Calorimeter
In a mixture of gases, each gas has a partial pressure which is the hypothetical pressure of that gas if it alone occupied the volume of the mixture at the same temperature.[1] The total pressure of an ideal gas mixture is the sum of the partial pressures of each individual gas in the mixture.
It relies on the following isotherm relation:
-
- Vx is the partial volume of any individual gas component (X)
- Vtot is the total volume in gas mixture
- px is the partial pressure of gas X
- ptot is the total pressure of gas mixture
- nx is the amount of substance of a gas (X)
- ntot is the total amount of substance in gas mixture
The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures, and not according to their concentrations in gas mixtures or liquids.
This general property of gases is also true of chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases, or dissolved in blood.
Dalton's law of partial pressures
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church.
The total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases in the mixture as stated by Dalton's law.[2] This is because ideal gas molecules are so far apart that they don't interact with each other. Most actual real-world gases come very close to this ideal. For example, given an ideal gas mixture of nitrogen (N2), hydrogen (H2) and ammonia (NH3):
| where: | |
| = total pressure of the gas mixture | |
| = partial pressure of nitrogen (N2) | |
| = partial pressure of hydrogen (H2) | |
| = partial pressure of ammonia (NH3) |
Ideal gas mixtures
Ideally the ratio of partial pressures equals the ratio of the number of molecules. That is, the mole fraction of an individual gas component in an ideal gas mixture can be expressed in terms of the component's partial pressure or the moles of the component:
and the partial pressure of an individual gas component in an ideal gas can be obtained using this expression:
The mole fraction of a gas component in a gas mixture is equal to the volumetric fraction of that component in a gas mixture.[3]
Partial volume (Amagat's law of additive volume)
The partial volume of a particular gas in a mixture is the volume of one component of the gas mixture. It is useful in gas mixtures, e.g. air, to focus on one particular gas component, e.g. oxygen.
It can be approximated both from partial pressure and molar fraction:[4]
-
- Vx is the partial volume of any individual gas component (X)
- Vtot is the total volume in gas mixture
- px is the partial pressure of gas X
- ptot is the total pressure of gas mixture
- nx is the amount of substance of a gas (X)
- ntot is the total amount of substance in gas mixture
Vapour pressure
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church.
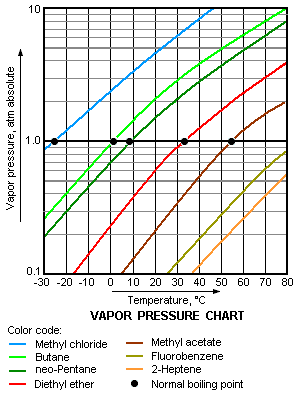
Vapour pressure is the pressure of a vapor in equilibrium with its non-vapor phases (i.e., liquid or solid). Most often the term is used to describe a liquid's tendency to evaporate. It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid. A liquid's atmospheric pressure boiling point corresponds to the temperature at which its vapour pressure is equal to the surrounding atmospheric pressure and it is often called the normal boiling point.
The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point of the liquid.
The vapour pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids.[5] As can be seen in the chart, the liquids with the highest vapour pressures have the lowest normal boiling points.
For example, at any given temperature, methyl chloride has the highest vapour pressure of any of the liquids in the chart. It also has the lowest normal boiling point (-24.2 °C), which is where the vapour pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapour pressure.
Equilibrium constants of reactions involving gas mixtures
It is possible to work out the equilibrium constant for a chemical reaction involving a mixture of gases given the partial pressure of each gas and the overall reaction formula. For a reversible reaction involving gas reactants and gas products, such as:
the equilibrium constant of the reaction would be:
For reversible reactions, changes in the total pressure, temperature or reactant concentrations will shift the equilibrium so as to favor either the right or left side of the reaction in accordance with Le Chatelier's Principle. However, the reaction kinetics may either oppose or enhance the equilibrium shift. In some cases, the reaction kinetics may be the over-riding factor to consider.
Henry's Law and the solubility of gases
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church.
Gases will dissolve in liquids to an extent that is determined by the equilibrium between the undissolved gas and the gas that has dissolved in the liquid (called the solvent).[6] The equilibrium constant for that equilibrium is:
where: = the equilibrium constant for the solvation process = partial pressure of gas in equilibrium with a solution containing some of the gas = the concentration of gas in the liquid solution
The form of the equilibrium constant shows that the concentration of a solute gas in a solution is directly proportional to the partial pressure of that gas above the solution. This statement is known as Henry's Law and the equilibrium constant is quite often referred to as the Henry's Law constant.[6][7][8]
Henry's Law is sometimes written as:[9]
where is also referred to as the Henry's Law constant.[9] As can be seen by comparing equations (1) and (2) above, is the reciprocal of . Since both may be referred to as the Henry's Law constant, readers of the technical literature must be quite careful to note which version of the Henry's Law equation is being used.
Henry's Law is an approximation that only applies for dilute, ideal solutions and for solutions where the liquid solvent does not react chemically with the gas being dissolved.
Partial pressure in diving breathing gases
In recreational diving and professional diving the richness of individual component gases of breathing gases is expressed by partial pressure.
Using diving terms, partial pressure is calculated as:
- partial pressure = (total absolute pressure) × (volume fraction of gas component)
For the component gas "i":
- ppi = P × Fi
For example, at 50 metres (165 feet), the total absolute pressure is 6 bar (600 kPa) (i.e., 1 bar of atmospheric pressure + 5 bar of water pressure) and the partial pressures of the main components of air, oxygen 21% by volume and nitrogen 79% by volume are:
- ppN2 = 6 bar × 0.79 = 4.7 bar absolute
- ppO2 = 6 bar × 0.21 = 1.3 bar absolute
The minimum safe lower limit for the partial pressures of oxygen in a gas mixture is 0.16 bar (16 kPa) absolute. Hypoxia and sudden unconsciousness becomes a problem with an oxygen partial pressure of less than 0.16 bar absolute. Oxygen toxicity, involving convulsions, becomes a problem when oxygen partial pressure is too high. The NOAA Diving Manual recommends a maximum single exposure of 45 minutes at 1.6 bar absolute, of 120 minutes at 1.5 bar absolute, of 150 minutes at 1.4 bar absolute, of 180 minutes at 1.3 bar absolute and of 210 minutes at 1.2 bar absolute. Oxygen toxicity becomes a risk when these oxygen partial pressures and exposures are exceeded. The partial pressure of oxygen determines the maximum operating depth of a gas mixture.
Nitrogen narcosis is a problem when breathing gases at high pressure. Typically, the maximum total partial pressure of narcotic gases used when planning for technical diving is 4.5 bar absolute, based on an equivalent narcotic depth of Template:Convert.
In medicine
The partial pressures of particularly oxygen () and carbon dioxide () are important parameters in tests of arterial blood gases, but can also be measured in, for example, cerebrospinal fluid.
| Unit | Arterial blood gas | Venous blood gas | Cerebrospinal fluid | Alveolar pulmonary gas pressures | |
|---|---|---|---|---|---|
| kPa | 11–13[10] | 4.0–5.3[10] | 5.3–5.9[10] | 14.2 | |
| mmHg | 75–100[11] | 30–40[12] | 40–44[13] | 107 | |
| kPa | 4.7–6.0[10] | 5.5–6.8[10] | 5.9–6.7[10] | 4.8 | |
| mmHg | 35–45[11] | 41–51[12] | 44–50[13] | 36 |
See also
Sportspersons Hyslop from Nicolet, usually spends time with pastimes for example martial arts, property developers condominium in singapore singapore and hot rods. Maintains a trip site and has lots to write about after touring Gulf of Porto: Calanche of Piana.
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
Template:Diving medicine, physiology and physics
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ Dalton's Law of Partial Pressures
- ↑ Frostberg State University's "General Chemistry Online"
- ↑ Page 200 in: Medical biophysics. Flemming Cornelius. 6th Edition, 2008.
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 6.0 6.1 An extensive list of Henry's law constants, and a conversion tool
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ Introductory University Chemistry, Henry's Law and the Solubility of Gases
- ↑ 9.0 9.1 University of Arizona chemistry class notes
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Derived from mmHg values using 0.133322 kPa/mmHg
- ↑ 11.0 11.1 Normal Reference Range Table from The University of Texas Southwestern Medical Center at Dallas. Used in Interactive Case Study Companion to Pathologic basis of disease.
- ↑ 12.0 12.1 The Medical Education Division of the Brookside Associates--> ABG (Arterial Blood Gas) Retrieved on Dec 6, 2009
- ↑ 13.0 13.1 PATHOLOGY 425 CEREBROSPINAL FLUID [CSF] at the Department of Pathology and Laboratory Medicine at the University of British Columbia. By Dr. G.P. Bondy. Retrieved November 2011









































