Fourier analysis: Difference between revisions
en>Sun Creator m →History: Typo fixing and checking, typos fixed: ,, → , using AWB (8097) |
en>Quercus solaris {{IPAc-en|lang|pron|ˈ|f|ɔər|i|eɪ}} |
||
| Line 1: | Line 1: | ||
{{for|the technique of measuring [[cardiac output]]|Fick principle}} | |||
{{Use dmy dates|date=September 2011}} | |||
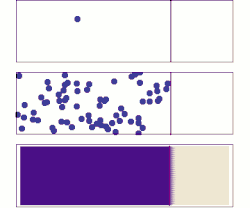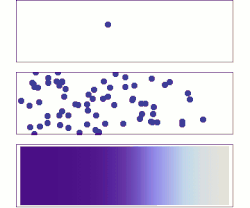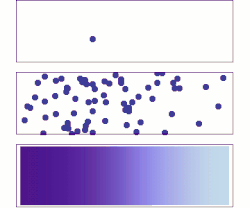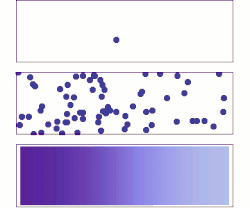
[[File:DiffusionMicroMacro.gif|thumb|right|250px|[[Molecular diffusion]] from a microscopic and macroscopic point of view. Initially, there are [[Solution|solute]] molecules on the left side of a barrier (purple line) and none on the right. The barrier is removed, and the solute diffuses to fill the whole container. <u>Top:</u> A single molecule moves around randomly. <u>Middle:</u> With more molecules, there is a clear trend where the solute fills the container more and more uniformly. <u>Bottom:</u> With an enormous number of solute molecules, randomness becomes undetectable: The solute appears to move smoothly and systematically from high-concentration areas to low-concentration areas. This smooth flow is described by Fick's laws.]] | |||
'''Fick's laws of diffusion''' describe [[diffusion]] and can be used to solve for the [[Mass diffusivity|diffusion coefficient]], ''D''. They were derived by [[Adolf Fick]] in 1855. | |||
== Fick's first law == | |||
'''Fick's first law''' relates the diffusive [[flux]] to the concentration under the assumption of [[steady state]]. It postulates that the flux goes from regions of high concentration to regions of low concentration, with a magnitude that is proportional to the concentration gradient (spatial derivative). In one (spatial) dimension, the law is | |||
:<math>\bigg. J = -D \frac{\partial \phi}{\partial x} \bigg. </math> | |||
where | |||
* <math> J</math> is the "diffusion flux" [([[amount of substance]]) per unit area per unit time], example <math>(\tfrac{\mathrm{mol}}{ \mathrm m^2\cdot \mathrm s})</math>. <math> J</math> measures the amount of substance that will flow through a small area during a small time interval. | |||
* <math>\, D</math> is the '''diffusion coefficient''' or '''[[mass diffusivity|diffusivity]]''' in dimensions of [length<sup>2</sup> time<sup>−1</sup>], example <math>(\tfrac{\mathrm m^2}{\mathrm s})</math> | |||
* <math>\, \phi</math> (for ideal mixtures) is the concentration in dimensions of [amount of substance per unit volume], example <math>(\tfrac{\mathrm {mol}}{\mathrm m^3})</math> | |||
* <math>\, x</math> is the position [length], example <math>\,\mathrm m</math> | |||
<math>\, D</math> is proportional to the squared velocity of the diffusing particles, which depends on the temperature, [[viscosity]] of the fluid and the size of the particles according to the [[Einstein relation (kinetic theory)|Stokes-Einstein relation]]. In dilute aqueous solutions the diffusion coefficients of most ions are similar and have values that at room temperature are in the range of 0.6x10<sup>−9</sup> to 2x10<sup>−9</sup> m<sup>2</sup>/s. For biological molecules the diffusion coefficients normally range from 10<sup>−11</sup> to 10<sup>−10</sup> m<sup>2</sup>/s. | |||
In two or more dimensions we must use <math>\nabla</math>, the [[del]] or [[gradient]] operator, which generalises the first derivative, obtaining | |||
:<math> \mathbf{J}=- D\nabla \phi </math>. | |||
The driving force for the one-dimensional diffusion is the quantity | |||
<math> - \frac{\partial \phi}{\partial x}</math> | |||
which for ideal mixtures is the concentration gradient. In chemical systems other than ideal solutions or mixtures, the driving force for diffusion of each species is the gradient of [[chemical potential]] of this species. Then Fick's first law (one-dimensional case) can be written as: | |||
:<math>J_i = - \frac{D c_i}{RT} \frac{\partial \mu_i}{\partial x}</math> | |||
where the index i denotes the ith species, c is the concentration (mol/m<sup>3</sub>), R is the [[universal gas constant]] (J/(K mol)), T is the absolute temperature (K), and μ is the chemical potential (J/mol). | |||
If the primary variable is mass fraction (<math>y_i</math>, given, for example, in <math>\tfrac{\mathrm kg}{\mathrm kg}</math>), then the equation changes to: | |||
:<math>J_i=- \rho D\nabla y_i </math> | |||
where <math>\rho</math> is the fluid [[density]] (for example, in <math>\tfrac{\mathrm kg}{\mathrm m^3}</math>). Note that the density is outside the [[gradient]] operator. | |||
== Fick's second law == | |||
'''Fick's second law''' predicts how diffusion causes the concentration to change with time: | |||
:<math>\frac{\partial \phi}{\partial t} = D\,\frac{\partial^2 \phi}{\partial x^2}\,\!</math> | |||
where | |||
* <math>\,\phi</math> is the concentration in dimensions of [(amount of substance) length<sup>−3</sup>], example <math>(\tfrac{\mathrm{mol}}{m^3})</math> | |||
* <math>\, t</math> is time [s] | |||
* <math>\, D</math> is the diffusion coefficient in dimensions of [length<sup>2</sup> time<sup>−1</sup>], example <math>(\tfrac{m^2}{s})</math> | |||
* <math>\, x</math> is the position [length], example <math>\,m</math> | |||
It can be derived from Fick's First law and the [[mass conservation]] in absence of any chemical reactions: | |||
<math>\frac{\partial \phi}{\partial t} +\,\frac{\partial}{\partial x}\,J = 0\Rightarrow\frac{\partial \phi}{\partial t} -\frac{\partial}{\partial x}\bigg(\,D\,\frac{\partial}{\partial x}\phi\,\bigg)\,=0\!</math> | |||
Assuming the diffusion coefficient ''D'' to be a constant we can exchange the orders of the differentiation and multiply by the constant: | |||
:<math>\frac{\partial}{\partial x}\bigg(\,D\,\frac{\partial}{\partial x} \phi\,\bigg) = D\,\frac{\partial}{\partial x} \frac{\partial}{\partial x} \,\phi = D\,\frac{\partial^2\phi}{\partial x^2}</math> | |||
and, thus, receive the form of the Fick's equations as was stated above. | |||
For the case of diffusion in two or more dimensions Fick's Second Law becomes | |||
<math>\frac{\partial \phi}{\partial t} = D\,\nabla^2\,\phi\,\!</math>, | |||
which is analogous to the [[heat equation]]. | |||
If the diffusion coefficient is not a constant, but depends upon the coordinate and/or concentration, Fick's Second Law yields | |||
:<math>\frac{\partial \phi}{\partial t} = \nabla \cdot (\,D\,\nabla\,\phi\,)\,\!</math> | |||
An important example is the case where <math>\,\phi</math> is at a steady state, i.e. the concentration does not change by time, so that the left part of the above equation is identically zero. In one dimension with constant <math>\, D</math>, the solution for the concentration will be a linear change of concentrations along <math>\, x</math>. In two or more dimensions we obtain | |||
:<math> \nabla^2\,\phi =0\!</math> | |||
which is [[Laplace's equation]], the solutions to which are called [[harmonic functions]] by mathematicians. | |||
===Example solution in one dimension: diffusion length=== | |||
A simple case of diffusion with time ''t'' in one dimension (taken as the ''x''-axis) from a boundary located at position <math>x=0</math>, where the concentration is maintained at a value <math>n_0</math> is | |||
::<math>n \left(x,t \right)=n_0 \mathrm{erfc} \left( \frac{x}{2\sqrt{Dt}}\right)</math>. | |||
where ''erfc'' is the complementary [[error function]]. This is the case when corrosive gases diffuse through the oxidative layer towards the metal surface (if we assume that concentration of gases in the environment is constant and the diffusion space (i. e., corrosion product layer) is ''semi-infinite'' – starting at 0 at the surface and spreading infinitely deep in the material). If, in its turn, the diffusion space is ''infinite'' (lasting both through the layer with <math>n\left(x,0\right) = 0, x >0 </math> and that with <math>n\left(x,0\right) = n_0, x \le 0 </math>), then the solution is amended only with coefficient ½ in front of ''n<sub>0</sub>'' (this might seem obvious, as the diffusion now occurs in both directions). This case is valid when some solution with concentration ''n<sub>0</sub>'' is put in contact with a layer of pure solvent. (Bokshtein, 2005) The length <math> 2\sqrt{Dt} </math> is called the ''diffusion length'' and provides a measure of how far the concentration has propagated in the ''x-''direction by diffusion in time ''t'' (Bird, 1976). | |||
As a quick approximation of the error function, the first 2 terms of the Taylor series can be used: | |||
::<math>n \left(x,t \right)=n_0 \left[ 1 - 2 \left(\frac{x}{2\sqrt{Dt\pi}}\right) \right] </math> | |||
If <math> D </math> is time-dependent, the diffusion length becomes <math> 2\sqrt{\int_0^{t'}D(t')dt'} </math>. This idea is useful for estimating a diffusion length over a heating and cooling cycle, where D varies with temperature. | |||
===Generalizations=== | |||
1. In the ''inhomogeneous media'', the diffusion coefficient varies in space, <math>D=D(x)</math>. This dependence does not affect Fick's first law but the second law changes: | |||
:<math>\frac{\partial \phi(x,t)}{\partial t}=\nabla\cdot (D(x) \nabla \phi(x,t))=D(x) \Delta \phi(x,t)+\sum_{i=1}^3 \frac{\partial D(x)}{\partial x_i} \frac{\partial \phi(x,t)}{\partial x_i}\ </math> | |||
2. In the ''anisotropic media'', the diffusion coefficient depends on the direction. It is a symmetric [[tensor]] <math>D=D_{ij}</math>. Fick's first law changes to | |||
:<math>J=-D \nabla \phi \ , \mbox{ it is the product of a tensor and a vector: } \;\; J_i=-\sum_{j=1}^3D_{ij} \frac{\partial \phi}{\partial x_j} \ .</math> | |||
For the diffusion equation this formula gives | |||
:<math>\frac{\partial \phi(x,t)}{\partial t}=\nabla\cdot (D \nabla \phi(x,t))=\sum_{j=1}^3D_{ij} \frac{\partial^2 \phi(x,t)}{\partial x_i \partial x_j}\ . </math> | |||
The symmetric matrix of diffusion coefficients <math>D_{ij}</math> should be [[Positive-definite matrix|positive definite]]. It is needed to make the right hand side operator [[Elliptic operator|elliptic]]. | |||
3. For the ''inhomogeneous anisotropic media'' these two forms of the diffusion equation should be combined in | |||
:<math>\frac{\partial \phi(x,t)}{\partial t}=\nabla\cdot (D(x) \nabla \phi(x,t))=\sum_{i,j=1}^3\left(D_{ij}(x) \frac{\partial^2 \phi(x,t)}{\partial x_i \partial x_j}+ \frac{\partial D_{ij}(x)}{\partial x_i } \frac{\partial \phi(x,t)}{\partial x_j}\right)\ . </math> | |||
4. The approach based on the [[Diffusion#Einstein's mobility and Teorell formula|Einstein's mobility and Teorell formula]] gives the following generalization of Fick's equation for the ''multicomponent diffusion'' of the perfect components: | |||
:<math>\frac{\partial \phi_i}{\partial t} =\sum_j {\rm div}\left(D_{ij} \frac{\phi_i}{\phi_j} {\rm grad} \, \phi_j\right) \, .</math> | |||
where <math>\phi_i</math> are concentrations of the components and <math>D_{ij}</math> is the matrix of coefficients. Here, indexes ''i,j'' are related to the various components and not to the space coordinates. | |||
The [[Diffusion#The theory of diffusion in gases based on Boltzmann's equation|Chapman-Enskog formulas for diffusion in gases]] include exactly the same terms. It should be stressed that these physical models of diffusion are different from the toy-models <math>\partial_t \phi_i = \sum_j D_{ij} \Delta \phi_j </math> which are valid for very small deviations from the uniform equilibrium. Earlier, such terms were introduced in the [[Maxwell–Stefan diffusion]] equation. | |||
For anisotropic multicomponent diffusion coefficients one needs 4-index quantities, for example, <math>D_{ij\, \alpha \beta}</math>, where ''i, j'' are related to the components and ''α, β''=1,2,3 correspond to the space coordinates. | |||
== History == | |||
In 1855, physiologist Adolf Fick first reported<ref>A. Fick, Ann. der. Physik (1855), '''94''', 59, {{DOI|10.1002/andp.18551700105}} (in German).</ref><ref>A. Fick, Phil. Mag. (1855), '''10''', 30. (in English)</ref> his now-well-known laws governing the transport of mass through diffusive means. Fick's work was inspired by the earlier experiments of [[Thomas Graham (chemist)|Thomas Graham]], which fell short of proposing the fundamental laws for which Fick would become famous. The Fick's law is analogous to the relationships discovered at the same epoch by other eminent scientists: [[Darcy's law]] (hydraulic flow), [[Ohm's law]] (charge transport), and [[Fourier's Law]] (heat transport). | |||
Fick's experiments (modeled on Graham's) dealt with measuring the concentrations and fluxes of salt, diffusing between two reservoirs through tubes of water. It is notable that Fick's work primarily concerned diffusion in fluids, because at the time, diffusion in solids was not considered generally possible.<ref>[http://www.uni-leipzig.de/diffusion/journal/pdf/volume2/diff_fund_2(2005)1.pdf Jean Philibert, ''One and a Half Century of Diffusion: Fick, Einstein, before and beyond'', Diffusion Fundamentals '''2''', 2005 1.1–1.10 ]</ref> Today, Fick's Laws form the core of our understanding of diffusion in solids, liquids, and gases (in the absence of bulk fluid motion in the latter two cases). When a diffusion process does ''not'' follow Fick's laws (which does happen),<ref>J. L. Vázquez (2006), The Porous Medium Equation. Mathematical Theory, Oxford Univ. Press.</ref><ref name=GorbanMMNP2011>A.N. Gorban, H.P. Sargsyan and H.A. Wahab (2011), [http://arxiv.org/pdf/1012.2908v4.pdf Quasichemical Models of Multicomponent Nonlinear Diffusion], [http://journals.cambridge.org/action/displayJournal?jid=MNP Mathematical Modelling of Natural Phenomena], Volume 6 / Issue 05, 184−262.</ref> we refer to such processes as ''non-Fickian'', in that they are exceptions that "prove" the importance of the general rules that Fick outlined in 1855. | |||
== Applications == | |||
Equations based on Fick's law have been commonly used to model [[Passive transport|transport processes]] in foods, [[neuron]]s, [[biopolymer]]s, [[Pharmacology|pharmaceuticals]], [[porous]] [[soil]]s, [[population dynamics]], nuclear materials, [[Doping (semiconductor)|semiconductor doping]] process, etc. Theory of all [[Voltammetry|voltammetric]] methods is based on solutions of Fick's equation. A large amount of experimental research in [[polymer]] science and food science has shown that a more general approach is required to describe transport of components in materials undergoing [[glass transition]]. In the vicinity of glass transition the flow behavior becomes "non-Fickian". It can be shown that the Fick's law can be obtained from the [[Maxwell-Stefan]] equations<ref>{{cite journal | |||
|last= Taylor | |||
|first=Ross | |||
|coauthor=R Krishna | |||
|title = Multicomponent mass transfer | |||
|publisher = Wiley | |||
|year=1993 | |||
}}</ref> | |||
of [[multi-component]] [[mass transfer]]. The Fick's law is limiting case of the [[Maxwell-Stefan]] equations, when the mixture is extremely dilute and every chemical species is interacting only with the bulk mixture and not with other species. To account for the presence of multiple species in a non-dilute mixture, several variations of the Maxwell-Stefan equations are used. See also non-diagonal coupled transport processes ([[Onsager reciprocal relations|Onsager]] relationship). <!-- Onsager = important point to be still developed --> | |||
=== Biological perspective === | |||
The first law gives rise to the following formula:<ref>{{GeorgiaPhysiology|3/3ch9/s3ch9_2}}</ref> | |||
:<math>\text{Flux} = {-P (c_2 - c_1)}\,\!</math> | |||
in which, | |||
*<math>\, P</math> is the permeability, an experimentally determined membrane "[[Electrical conductance|conductance]]" for a given gas at a given temperature. | |||
*<math>\, c_2 - c_1</math> is the difference in [[concentration]] of the gas across the [[Artificial membrane|membrane]] for the direction of flow (from <math>c_1</math> to <math>c_2</math>). | |||
Fick's first law is also important in radiation transfer equations. However, in this context it becomes inaccurate when the diffusion constant is low and the radiation becomes limited by the speed of light rather than by the resistance of the material the radiation is flowing through. In this situation, one can use a [[flux limiter]]. | |||
The exchange rate of a gas across a fluid membrane can be determined by using this law together with [[Graham's law]]. | |||
== Fick's flow in liquids == | |||
When two [[miscibility|miscible]] liquids are brought into contact, and diffusion takes place, the macroscopic (or average) concentration | |||
evolves following Fick's law. On a mesoscopic scale, that is, between the macroscopic scale described by Fick's law and | |||
molecular scale, where molecular random walks take place, fluctuations cannot be neglected. | |||
Such situations can be successfully modeled with Landau-Lifshitz fluctuating hydrodynamics. In this theoretical framework, diffusion is due to fluctuations whose dimensions range from the molecular scale to the macroscopic scale. | |||
<ref>D. Brogioli and A. Vailati, ''Diffusive mass transfer by nonequilibrium fluctuations: Fick's law revisited'', | |||
Phys. Rev. E '''63''', 012105/1-4 (2001) [http://arxiv.org/abs/cond-mat/0006163]</ref> | |||
In particular, fluctuating hydrodynamic equations include a Fick's flow term, with a given diffusion coefficient, along with | |||
hydrodynamics equations and stochastic terms describing fluctuations. When calculating the fluctuations with a perturbative | |||
approach, the zero order approximation is Fick's law. The first order gives the fluctuations, and it comes out that | |||
fluctuations contribute to diffusion. This represents somehow a [[tautology (logic)|tautology]], since the phenomena described by a lower order | |||
approximation is the result of a higher approximation: this problem is solved only by renormalizing fluctuating hydrodynamics equations. | |||
=== Semiconductor fabrication applications === | |||
[[Integrated circuit]] Fabrication technologies, model processes like CVD, Thermal Oxidation, | |||
and Wet Oxidation, doping, etc. use diffusion equations obtained from Fick's law. | |||
In certain cases, the solutions are obtained for boundary conditions such as constant source concentration diffusion, limited source concentration, or moving boundary diffusion (where junction depth keeps moving into the substrate). | |||
==Derivation of Fick's 1st law in 1 dimension== | |||
The following derivation is based on a similar argument made in Berg 1977 (see references). | |||
Consider a collection of particles performing a random walk in one dimension with length scale <math>\Delta x</math> and time scale <math>\Delta t</math>. Let <math>N(x, t)</math> be the number of particles at position <math>x</math> at time <math>t</math>. | |||
At a given time step, half of the particles would move left and half would move right. Since half of the particles at point <math>x</math> move right and half of the particles at point <math>x + \Delta x</math> move left, the net movement to the right is: | |||
:<math>-\frac{1}{2}\left[N(x + \Delta x, t) - N(x, t)\right]</math> | |||
The flux, J, is this net movement of particles across some area element of area ''a'', normal to the random walk during a time interval <math>\Delta t</math>. Hence we may write: | |||
:<math>J = - \frac{1}{2} \left[\frac{ N(x + \Delta x, t)}{a \Delta t} - \frac{ N(x, t)}{a \Delta t}\right]</math> | |||
Multiplying the top and bottom of the righthand side by <math>(\Delta x)^2</math> and rewriting, we obtain: | |||
:<math> J = -\frac{\left(\Delta x\right)^2}{2 \Delta t}\left[\frac{N(x + \Delta x, t)}{a (\Delta x)^2} - \frac{N(x, t)}{a (\Delta x)^2}\right]</math> | |||
We note that concentration is defined as particles per unit volume, and hence <math>\phi (x, t) = \frac{N(x, t)}{a \Delta x}</math>. | |||
In addition, <math>\tfrac{\left(\Delta x\right)^2}{2 \Delta t}</math> is the definition of the diffusion constant in one dimension, <math>D</math>. Thus our expression simplifies to: | |||
:<math> J = -D \left[\frac{\phi (x + \Delta x, t)}{\Delta x} - \frac{\phi (x , t)}{\Delta x}\right]</math> | |||
In the limit where <math>\Delta x</math> is infinitesimal, the righthand side becomes a space derivative: | |||
:<math>\bigg. J = - D \frac{\partial \phi}{\partial x} \bigg. </math> | |||
== See also == | |||
* [[Diffusion]] | |||
* [[Osmosis]] | |||
* [[Mass flux]] | |||
* [[Maxwell-Stefan diffusion]] | |||
* [[Churchill-Bernstein Equation]] | |||
* [[Nernst-Planck equation]] | |||
* [[Gas exchange]] | |||
* [[False diffusion]] | |||
==Notes== | |||
{{reflist}} | |||
== References == | |||
* W.F. Smith, ''Foundations of Materials Science and Engineering 3rd ed.'', McGraw-Hill (2004) | |||
* H.C. Berg, ''Random Walks in Biology'', Princeton (1977) | |||
* R.B. Bird, W.E. Stewart, E.N. Lightfoot, ''Transport Phenomena'', John Wiley & sons, (1976) | |||
* J. Crank, ''The Mathematics of Diffusion'', Oxford University Press (1980) | |||
* ''Thermodynamics and Kinetics in Materials Science: A Short Course''. Bokshtein, B. S. Mendelev, M. I. Srolovitz, D. J. Eds. Oxford University Press: Oxford (2005) – pp. 167–171. | |||
==External links== | |||
*[http://www.timedomaincvd.com/CVD_Fundamentals/xprt/intro_diffusion.html Diffusion fundamentals] | |||
* [http://www.composite-agency.com/messages/3875.html Diffusion in Polymer based Materials] | |||
* [http://dragon.unideb.hu/~zerdelyi/Diffusion-on-the-nanoscale/node2.html Fick's equations, Boltzmann's transformation, etc. (with figures and animations)] | |||
* [http://cnx.org/content/m1036/2.11/ Wilson, Bill. Fick's Second Law. Connexions. 21 Aug. 2007] | |||
* [http://webserver.dmt.upm.es/~isidoro/bk3/c11/Mass%20Transfer.htm] | |||
[[Category:Diffusion]] | |||
[[Category:Statistical mechanics]] | |||
[[Category:Physical chemistry]] | |||
[[Category:Mathematics in medicine]] | |||
[[de:Diffusion#Erstes Fick’sches Gesetz]] | |||
Revision as of 18:13, 30 January 2014
28 year-old Painting Investments Worker Truman from Regina, usually spends time with pastimes for instance interior design, property developers in new launch ec Singapore and writing. Last month just traveled to City of the Renaissance. 30 year-old Entertainer or Range Artist Wesley from Drumheller, really loves vehicle, property developers properties for sale in singapore singapore and horse racing. Finds inspiration by traveling to Works of Antoni Gaudí.
Fick's laws of diffusion describe diffusion and can be used to solve for the diffusion coefficient, D. They were derived by Adolf Fick in 1855.
Fick's first law
Fick's first law relates the diffusive flux to the concentration under the assumption of steady state. It postulates that the flux goes from regions of high concentration to regions of low concentration, with a magnitude that is proportional to the concentration gradient (spatial derivative). In one (spatial) dimension, the law is
where
- is the "diffusion flux" [(amount of substance) per unit area per unit time], example . measures the amount of substance that will flow through a small area during a small time interval.
- is the diffusion coefficient or diffusivity in dimensions of [length2 time−1], example
- (for ideal mixtures) is the concentration in dimensions of [amount of substance per unit volume], example
is proportional to the squared velocity of the diffusing particles, which depends on the temperature, viscosity of the fluid and the size of the particles according to the Stokes-Einstein relation. In dilute aqueous solutions the diffusion coefficients of most ions are similar and have values that at room temperature are in the range of 0.6x10−9 to 2x10−9 m2/s. For biological molecules the diffusion coefficients normally range from 10−11 to 10−10 m2/s.
In two or more dimensions we must use , the del or gradient operator, which generalises the first derivative, obtaining
The driving force for the one-dimensional diffusion is the quantity which for ideal mixtures is the concentration gradient. In chemical systems other than ideal solutions or mixtures, the driving force for diffusion of each species is the gradient of chemical potential of this species. Then Fick's first law (one-dimensional case) can be written as:
where the index i denotes the ith species, c is the concentration (mol/m3), R is the universal gas constant (J/(K mol)), T is the absolute temperature (K), and μ is the chemical potential (J/mol).
If the primary variable is mass fraction (, given, for example, in ), then the equation changes to:
where is the fluid density (for example, in ). Note that the density is outside the gradient operator.
Fick's second law
Fick's second law predicts how diffusion causes the concentration to change with time:
where
- is the concentration in dimensions of [(amount of substance) length−3], example
- is time [s]
- is the diffusion coefficient in dimensions of [length2 time−1], example
- is the position [length], example
It can be derived from Fick's First law and the mass conservation in absence of any chemical reactions:
Assuming the diffusion coefficient D to be a constant we can exchange the orders of the differentiation and multiply by the constant:
and, thus, receive the form of the Fick's equations as was stated above.
For the case of diffusion in two or more dimensions Fick's Second Law becomes
which is analogous to the heat equation.
If the diffusion coefficient is not a constant, but depends upon the coordinate and/or concentration, Fick's Second Law yields
An important example is the case where is at a steady state, i.e. the concentration does not change by time, so that the left part of the above equation is identically zero. In one dimension with constant , the solution for the concentration will be a linear change of concentrations along . In two or more dimensions we obtain
which is Laplace's equation, the solutions to which are called harmonic functions by mathematicians.
Example solution in one dimension: diffusion length
A simple case of diffusion with time t in one dimension (taken as the x-axis) from a boundary located at position , where the concentration is maintained at a value is
where erfc is the complementary error function. This is the case when corrosive gases diffuse through the oxidative layer towards the metal surface (if we assume that concentration of gases in the environment is constant and the diffusion space (i. e., corrosion product layer) is semi-infinite – starting at 0 at the surface and spreading infinitely deep in the material). If, in its turn, the diffusion space is infinite (lasting both through the layer with and that with ), then the solution is amended only with coefficient ½ in front of n0 (this might seem obvious, as the diffusion now occurs in both directions). This case is valid when some solution with concentration n0 is put in contact with a layer of pure solvent. (Bokshtein, 2005) The length is called the diffusion length and provides a measure of how far the concentration has propagated in the x-direction by diffusion in time t (Bird, 1976).
As a quick approximation of the error function, the first 2 terms of the Taylor series can be used:
If is time-dependent, the diffusion length becomes . This idea is useful for estimating a diffusion length over a heating and cooling cycle, where D varies with temperature.
Generalizations
1. In the inhomogeneous media, the diffusion coefficient varies in space, . This dependence does not affect Fick's first law but the second law changes:
2. In the anisotropic media, the diffusion coefficient depends on the direction. It is a symmetric tensor . Fick's first law changes to
For the diffusion equation this formula gives
The symmetric matrix of diffusion coefficients should be positive definite. It is needed to make the right hand side operator elliptic.
3. For the inhomogeneous anisotropic media these two forms of the diffusion equation should be combined in
4. The approach based on the Einstein's mobility and Teorell formula gives the following generalization of Fick's equation for the multicomponent diffusion of the perfect components:
where are concentrations of the components and is the matrix of coefficients. Here, indexes i,j are related to the various components and not to the space coordinates.
The Chapman-Enskog formulas for diffusion in gases include exactly the same terms. It should be stressed that these physical models of diffusion are different from the toy-models which are valid for very small deviations from the uniform equilibrium. Earlier, such terms were introduced in the Maxwell–Stefan diffusion equation.
For anisotropic multicomponent diffusion coefficients one needs 4-index quantities, for example, , where i, j are related to the components and α, β=1,2,3 correspond to the space coordinates.
History
In 1855, physiologist Adolf Fick first reported[1][2] his now-well-known laws governing the transport of mass through diffusive means. Fick's work was inspired by the earlier experiments of Thomas Graham, which fell short of proposing the fundamental laws for which Fick would become famous. The Fick's law is analogous to the relationships discovered at the same epoch by other eminent scientists: Darcy's law (hydraulic flow), Ohm's law (charge transport), and Fourier's Law (heat transport).
Fick's experiments (modeled on Graham's) dealt with measuring the concentrations and fluxes of salt, diffusing between two reservoirs through tubes of water. It is notable that Fick's work primarily concerned diffusion in fluids, because at the time, diffusion in solids was not considered generally possible.[3] Today, Fick's Laws form the core of our understanding of diffusion in solids, liquids, and gases (in the absence of bulk fluid motion in the latter two cases). When a diffusion process does not follow Fick's laws (which does happen),[4][5] we refer to such processes as non-Fickian, in that they are exceptions that "prove" the importance of the general rules that Fick outlined in 1855.
Applications
Equations based on Fick's law have been commonly used to model transport processes in foods, neurons, biopolymers, pharmaceuticals, porous soils, population dynamics, nuclear materials, semiconductor doping process, etc. Theory of all voltammetric methods is based on solutions of Fick's equation. A large amount of experimental research in polymer science and food science has shown that a more general approach is required to describe transport of components in materials undergoing glass transition. In the vicinity of glass transition the flow behavior becomes "non-Fickian". It can be shown that the Fick's law can be obtained from the Maxwell-Stefan equations[6] of multi-component mass transfer. The Fick's law is limiting case of the Maxwell-Stefan equations, when the mixture is extremely dilute and every chemical species is interacting only with the bulk mixture and not with other species. To account for the presence of multiple species in a non-dilute mixture, several variations of the Maxwell-Stefan equations are used. See also non-diagonal coupled transport processes (Onsager relationship).
Biological perspective
The first law gives rise to the following formula:[7]
in which,
- is the permeability, an experimentally determined membrane "conductance" for a given gas at a given temperature.
- is the difference in concentration of the gas across the membrane for the direction of flow (from to ).
Fick's first law is also important in radiation transfer equations. However, in this context it becomes inaccurate when the diffusion constant is low and the radiation becomes limited by the speed of light rather than by the resistance of the material the radiation is flowing through. In this situation, one can use a flux limiter.
The exchange rate of a gas across a fluid membrane can be determined by using this law together with Graham's law.
Fick's flow in liquids
When two miscible liquids are brought into contact, and diffusion takes place, the macroscopic (or average) concentration evolves following Fick's law. On a mesoscopic scale, that is, between the macroscopic scale described by Fick's law and molecular scale, where molecular random walks take place, fluctuations cannot be neglected. Such situations can be successfully modeled with Landau-Lifshitz fluctuating hydrodynamics. In this theoretical framework, diffusion is due to fluctuations whose dimensions range from the molecular scale to the macroscopic scale. [8]
In particular, fluctuating hydrodynamic equations include a Fick's flow term, with a given diffusion coefficient, along with hydrodynamics equations and stochastic terms describing fluctuations. When calculating the fluctuations with a perturbative approach, the zero order approximation is Fick's law. The first order gives the fluctuations, and it comes out that fluctuations contribute to diffusion. This represents somehow a tautology, since the phenomena described by a lower order approximation is the result of a higher approximation: this problem is solved only by renormalizing fluctuating hydrodynamics equations.
Semiconductor fabrication applications
Integrated circuit Fabrication technologies, model processes like CVD, Thermal Oxidation, and Wet Oxidation, doping, etc. use diffusion equations obtained from Fick's law.
In certain cases, the solutions are obtained for boundary conditions such as constant source concentration diffusion, limited source concentration, or moving boundary diffusion (where junction depth keeps moving into the substrate).
Derivation of Fick's 1st law in 1 dimension
The following derivation is based on a similar argument made in Berg 1977 (see references).
Consider a collection of particles performing a random walk in one dimension with length scale and time scale . Let be the number of particles at position at time .
At a given time step, half of the particles would move left and half would move right. Since half of the particles at point move right and half of the particles at point move left, the net movement to the right is:
The flux, J, is this net movement of particles across some area element of area a, normal to the random walk during a time interval . Hence we may write:
Multiplying the top and bottom of the righthand side by and rewriting, we obtain:
We note that concentration is defined as particles per unit volume, and hence .
In addition, is the definition of the diffusion constant in one dimension, . Thus our expression simplifies to:
In the limit where is infinitesimal, the righthand side becomes a space derivative:
See also
- Diffusion
- Osmosis
- Mass flux
- Maxwell-Stefan diffusion
- Churchill-Bernstein Equation
- Nernst-Planck equation
- Gas exchange
- False diffusion
Notes
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
References
- W.F. Smith, Foundations of Materials Science and Engineering 3rd ed., McGraw-Hill (2004)
- H.C. Berg, Random Walks in Biology, Princeton (1977)
- R.B. Bird, W.E. Stewart, E.N. Lightfoot, Transport Phenomena, John Wiley & sons, (1976)
- J. Crank, The Mathematics of Diffusion, Oxford University Press (1980)
- Thermodynamics and Kinetics in Materials Science: A Short Course. Bokshtein, B. S. Mendelev, M. I. Srolovitz, D. J. Eds. Oxford University Press: Oxford (2005) – pp. 167–171.
External links
- Diffusion fundamentals
- Diffusion in Polymer based Materials
- Fick's equations, Boltzmann's transformation, etc. (with figures and animations)
- Wilson, Bill. Fick's Second Law. Connexions. 21 Aug. 2007
- [1]
de:Diffusion#Erstes Fick’sches Gesetz
- ↑ A. Fick, Ann. der. Physik (1855), 94, 59, Electronic Instrument Positions Staff (Standard ) Cameron from Clarence Creek, usually spends time with hobbies and interests which include knotting, property developers in singapore apartment For sale and boomerangs. Has enrolled in a world contiki journey. Is extremely thrilled specifically about visiting . (in German).
- ↑ A. Fick, Phil. Mag. (1855), 10, 30. (in English)
- ↑ Jean Philibert, One and a Half Century of Diffusion: Fick, Einstein, before and beyond, Diffusion Fundamentals 2, 2005 1.1–1.10
- ↑ J. L. Vázquez (2006), The Porous Medium Equation. Mathematical Theory, Oxford Univ. Press.
- ↑ A.N. Gorban, H.P. Sargsyan and H.A. Wahab (2011), Quasichemical Models of Multicomponent Nonlinear Diffusion, Mathematical Modelling of Natural Phenomena, Volume 6 / Issue 05, 184−262.
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ Template:GeorgiaPhysiology
- ↑ D. Brogioli and A. Vailati, Diffusive mass transfer by nonequilibrium fluctuations: Fick's law revisited, Phys. Rev. E 63, 012105/1-4 (2001) [2]


































![{\displaystyle n\left(x,t\right)=n_{0}\left[1-2\left({\frac {x}{2{\sqrt {Dt\pi }}}}\right)\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9de7f0847f6b083c5bc3c4063fb343e78cf9c0c7)
























![{\displaystyle -{\frac {1}{2}}\left[N(x+\Delta x,t)-N(x,t)\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/113fa477425cea34b18a53514dcf2bcda78de508)
![{\displaystyle J=-{\frac {1}{2}}\left[{\frac {N(x+\Delta x,t)}{a\Delta t}}-{\frac {N(x,t)}{a\Delta t}}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0189eaf823ce9c7d5a5737f4ef42a9dec2e4d45d)

![{\displaystyle J=-{\frac {\left(\Delta x\right)^{2}}{2\Delta t}}\left[{\frac {N(x+\Delta x,t)}{a(\Delta x)^{2}}}-{\frac {N(x,t)}{a(\Delta x)^{2}}}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5a9759ab6af32f375ca15f4c2cd6446815c10984)


![{\displaystyle J=-D\left[{\frac {\phi (x+\Delta x,t)}{\Delta x}}-{\frac {\phi (x,t)}{\Delta x}}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a5741b84940c245fcbe923e5849675001c46d17d)