William Rowan Hamilton: Difference between revisions
en>EmausBot m r2.7.3) (Robot: Adding hr:William Rowan Hamilton |
en>Isarra (HG) m Reverted edits by 87.36.36.5 (talk): editing tests (HG) |
||
| Line 1: | Line 1: | ||
[[File:Vapor pressure.svg|thumb|The picture shows the particle transition, as a result of their vapor pressure, from the liquid phase to the gas phase and converse.]] | |||
'''Vapor pressure''' or '''equilibrium vapor pressure''' is defined as the [[pressure]] exerted by a [[vapor]] in [[thermodynamic equilibrium]] with its [[Condensation|condensed]] [[Phase (matter)|phase]]s (solid or liquid) at a given temperature in a [[Thermodynamic_system#Closed_system|closed system]]. The equilibrium vapor pressure is an indication of a liquid's [[evaporation]] rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as ''[[volatility (chemistry)|volatile]]''. | |||
The vapor pressure of any substance increases non-linearly with temperature according to the [[Clausius–Clapeyron relation]]. The [[atmospheric pressure]] [[boiling point]] of a liquid (also known as the [[normal boiling point]]) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome [[atmospheric pressure]] and lift the liquid to form vapor bubbles inside the bulk of the substance. [[liquid bubble|Bubble]] formation deeper in the liquid requires a higher pressure, and therefore higher temperature, because the fluid pressure increases above the atmospheric pressure as the depth increases. | |||
The vapor pressure that a single component in a mixture contributes to the total pressure in the system is called [[partial pressure]]. For example, air at sea level, and saturated with water vapor at 20 °C, has partial pressures of about 23 mbar of [[water]], 780 mbar of [[nitrogen]], 210 mbar of [[oxygen]] and 9 mbar of [[argon]]. | |||
==Measurement and units== | |||
Vapor pressure is measured in the standard units of [[pressure]]. The [[International System of Units]] (SI) recognizes pressure as a [[SI derived unit|derived unit]] with the dimension of force per area and designates the [[pascal (unit)|pascal]] (Pa) as its standard unit. One pascal is one [[newton (unit)|newton]] per [[square meter]] (N·m<sup>−2</sup> or kg·m<sup>−1</sup>·s<sup>−2</sup>). | |||
Experimental measurement of vapor pressure is a simple procedure for common pressures between 1 and 200 kPa.<ref>{{cite web|url=http://www.capec.kt.dtu.dk/documents/overview/Vapor-pressure-Ruzicka.pdf|title=Vapor Pressure of Organic Compounds. Measurement and Correlation|author=K. Růžička, M. Fulem, V. Růžička}}</ref> Most accurate results are obtained near the boiling point of substances and large errors result for measurements smaller than {{gaps|1|kPa}}. Procedures often consist of purifying the test substance, isolating it in a container, evacuating any foreign gas, then measuring the equilibrium pressure of the gaseous phase of the substance in the container at different temperatures. Better accuracy is achieved when care is taken to ensure that the entire substance and its vapor are at the prescribed temperature. This is often done, as with the use of an [[isoteniscope]], by submerging the containment area in a liquid bath. | |||
==Estimating vapor pressures with Antoine equation== | |||
The [[Antoine equation]] <ref name=frostburg>[http://antoine.frostburg.edu/chem/senese/101/liquids/faq/antoine-vapor-pressure.shtml What is the Antoine Equation?] (Chemistry Department, [[Frostburg State University]], [[Maryland]])</ref><ref name=Sinnot>{{cite book|author=R.K.Sinnot|title=[http://books.google.ca/books?id=DJaxUL3numgC&pg=PA331&lpg=PA331&dq=antoine+equation+constants&source=bl&ots=2c0cqzbR0t&sig=YPuvrW2kWnWP2s4QvY9TTpxGgNM&hl=en&sa=X&ei=widrUcu9Cce0qgHq24CgCA&ved=0CGcQ6AEwBzgK#v=onepage&q=antoine%20equation%20constants&f=false Chemical Engineering Design]|edition=4th|publisher=Butterworth-Heinemann|year=2005|page=331|isbn=0-7506-6538-6}}</ref> is a mathematical expression of the relation between the vapor pressure and the temperature of pure liquid or solid substances. The basic form of the equation is: | |||
:<math>\log P = A-\frac{B}{C+T}</math> | |||
and it can be transformed into this temperature-explicit form: | |||
:<math>T = \frac{B}{A-\log P} - C</math> | |||
where: <span style="vertical-align:+12%;"><math>P</math></span> is the absolute vapor pressure of a substance<br> | |||
: <span style="vertical-align:+15%;"><span style="vertical-align:+12%;"><math>T</math></span> is the temperature of the substance</span> | |||
: <span style="vertical-align:+12%;"><math>A</math></span>, <span style="vertical-align:+12%;"><math>B</math></span> and <span style="vertical-align:+12%;"><math>C</math></span> are substance-specific coefficients (i.e., constants or parameters) | |||
: <span style="vertical-align:-30%;"><math>\log</math> is typically either <math>\log_{10}</math> or <math>\log_e</math></span><ref name=Sinnot/> | |||
A simpler form of the equation with only two coefficients is sometimes used: | |||
:<math>\log P = A-\frac{B}{T}</math> | |||
which can be transformed to: | |||
:<math>T = \frac{B}{A-\log P}</math> | |||
Sublimations and vaporizations of the same substance have separate sets of Antoine coefficients, as do components in mixtures.<ref name=frostburg/> Each parameter set for a specific compound is only applicable over a specified temperature range. Generally, temperature ranges are chosen to maintain the equation's accuracy of a few up to 8-10 percent. For many volatile substances, several different sets of parameters are available and used for different temperature ranges. The Antoine equation has poor accuracy with any single parameter set when used from a compound's melting point to its critical temperature. Accuracy is also usually poor when vapor pressure is under 10 Torr because of the limitations of the apparatus used to establish the Antoine parameter values. | |||
The Wagner Equation<ref>{{Citation|last= Wagner|first= W.|title= New vapour pressure measurements for argon and nitrogen and an new method for establishing rational vapour pressure equations|journal= Cryogenics|volume= 13|issue= 8|pages= 470–482 |year= 1973|doi= }}</ref> gives "one of the best"<ref>Perry's Chemical Engineers' Handbook, 7th Ed. pg 4-15</ref> fits to experimental data but is quite complex. It expresses reduced vapor pressure as a function of reduced temperature. | |||
==Relation to boiling point of liquids== | |||
{{further|Boiling point}} | |||
[[Image:Vapor Pressure Chart.png|thumb|right|301 px|A typical vapor pressure chart for various liquids]] | |||
As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing boiling points. This is illustrated in the vapor pressure chart (see right) that shows graphs of the '''vapor pressures versus temperatures''' for a variety of liquids.<ref>{{cite book|author=Perry, R.H. and Green, D.W. (Editors)|title=[[Perry's Chemical Engineers' Handbook]]|edition=7th|publisher=McGraw-Hill|year=1997|isbn= 0-07-049841-5}}</ref> | |||
For example, at any given temperature, [[methyl chloride]] has the highest vapor pressure of any of the liquids in the chart. It also has the lowest normal boiling point (−24.2 °C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere ([[Atmosphere (unit)|atm]]) of absolute vapor pressure. | |||
Although the relation between vapor pressure and temperature is non-linear, the chart uses a logarithmic vertical axis to produce slightly curved lines, so one chart can graph many liquids. A nearly straight line is obtained when the logarithm of the vapor pressure is plotted against 1/(T+230)<ref>{{cite article|author=Dreisbach, R. R. and Spencer, R. S.| title=Infinite Points of Cox Chart Families and dt/dP Values at any Pressure|journal=Industrial and Engineering Chemistry,|volume=41|number=1|page=176|month=January|year=1949}}</ref> where T is the temperature in degrees Celsius. The vapor pressure of a liquid at its boiling point equals the pressure of its surrounding environment. | |||
==Liquid mixtures== | |||
[[Raoult's law]] gives an approximation to the vapor pressure of mixtures of liquids. It states that the activity (pressure or [[fugacity]]) of a single-phase mixture is equal to the mole-fraction-weighted sum of the components' vapor pressures: | |||
:<math> p_\text{tot} = \sum_i p_i \chi_i \,</math> | |||
where '''''p'''''<sub> '''tot'''</sub> is the mixture's vapor pressure, '''''i''''' is one of the components of the mixture and '''''Χ<sub>i</sub>''''' is the [[mole fraction]] of that component in the liquid mixture. The term '''''p<sub>i</sub>Χ<sub>i</sub>''''' is the partial pressure of component '''''i''''' in the mixture. Raoult's Law is applicable only to non-electrolytes (uncharged species); it is most appropriate for non-polar molecules with only weak intermolecular attractions (such as [[London force]]s). | |||
Systems that have vapor pressures higher than indicated by the above formula are said to have positive deviations. Such a deviation suggests weaker intermolecular attraction than in the pure components, so that the molecules can be thought of as being "held in" the liquid phase less strongly than in the pure liquid. An example is the [[azeotrope]] of approximately 95% ethanol and water. Because the azeotrope's vapor pressure is higher than predicted by Raoult's law, it boils at a temperature below that of either pure component. | |||
There are also systems with negative deviations that have vapor pressures that are lower than expected. Such a deviation is evidence for stronger intermolecular attraction between the constituents of the mixture than exists in the pure components. Thus, the molecules are "held in" the liquid more strongly when a second molecule is present. An example is a mixture of trichloromethane (chloroform) and 2-propanone (acetone), which boils above the boiling point of either pure component. | |||
The negative and positive deviations can be used to determine [[thermodynamic activity]] coefficients of the components of mixtures. | |||
==Solids== | |||
[[Image:Vapor Pressure Curve of Liquid and Solid Benzene.png|thumb|300px|Vapor pressure of liquid and solid benzene]] | |||
Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in equilibrium with its own vapor. In the case of an equilibrium solid, such as a [[crystal]], this can be defined as the pressure when the rate of [[sublimation (physics)|sublimation]] of a solid matches the rate of deposition of its vapor phase. For most solids this pressure is very low, but some notable exceptions are [[naphthalene]], [[dry ice]] (the vapor pressure of dry ice is 5.73 MPa (831 psi, 56.5 atm) at 20 degrees Celsius, which causes most sealed containers to rupture), and ice. All solid materials have a vapor pressure. However, due to their often extremely low values, measurement can be rather difficult. Typical techniques include the use of [[thermogravimetry]] and [[gas transpiration]]. | |||
There are a number of methods for calculating the sublimation pressure (i.e., the vapor pressure) of a solid. One method is to estimate the sublimation pressure from extrapolated liquid vapor pressures (of the supercooled liquid), if the [[Enthalpy of fusion|heat of fusion]] is known, by using this particular form of the [[Clausius–Clapeyron relation]]:<ref name="Moller">Moller B., Rarey J., Ramjugernath D., "Estimation of the vapour pressure of non-electrolyte organic compounds via group contributions and group interactions ", J.Mol.Liq., 143(1), 52-63, 2008</ref> | |||
:<math>\ln\,P^S_{solid} = \ln\,P^S_{liquid} - \frac{\Delta H_m}{R} \left( \frac{1}{T} - \frac{1}{T_m} \right)</math> | |||
with: | |||
{| border="0" cellpadding="1" | |||
|- | |||
!align=right|<math>P^S_{solid}</math> | |||
|align=left|= Sublimation pressure of the solid component at the temperature <math>T<T_m</math> | |||
|- | |||
!align=right|<math>P^S_{liquid}</math> | |||
|align=left|= Extrapolated vapor pressure of the liquid component at the temperature <math>T<T_m</math> | |||
|- | |||
!align=right|<math>\Delta H_m</math> | |||
|align=left|= Heat of fusion | |||
|- | |||
!align=right|<math>R</math> | |||
|align=left|= [[Gas constant]] | |||
|- | |||
!align=right|<math>T</math> | |||
|align=left|= Sublimation temperature | |||
|- | |||
!align=right|<math>T_m</math> | |||
|align=left|= Melting point temperature | |||
|} | |||
This method assumes that the heat of fusion is temperature-independent, ignores additional transition temperatures between different solid phases, and it gives a fair estimation for temperatures not too far from the melting point. It also shows that the sublimation pressure is lower than the extrapolated liquid vapor pressure (Δ''H''<sub>m</sub> is positive) and the difference grows with increased distance from the melting point. | |||
==Boiling point of water== | |||
[[Image:Water vapor pressure graph.jpg|thumb|right|Graph of water vapor pressure versus temperature. At the normal boiling point of 100°C, it equals the standard atmospheric pressure of 760 Torr or 101.325 kPa.]] | |||
{{main|Vapor pressure of water}} | |||
Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of [[water]] for atmospheric pressures can be approximated by the [[Antoine equation]]: | |||
:<math>\log_{10}P = 8.07131 - \frac{1730.63}{233.426 + T_b}</math> | |||
or transformed into this temperature-explicit form: | |||
:<math>T_b = \frac{1730.63}{8.07131 - \log_{10}P} - 233.426</math> | |||
where the temperature <math>T_b</math> is the boiling point in degrees [[Celsius]] and the pressure <math>P_{ } </math> is in [[Torr]]. | |||
==Dühring's rule== | |||
{{main|Dühring's rule}} | |||
Dühring's rule states that a linear relationship exists between the temperatures at which two solutions exert the same vapor pressure. | |||
==Examples== | |||
The following table is a list of a variety of substances ordered by increasing vapor pressure. | |||
{|style="text-align:center;" class="wikitable" | |||
! Substance | |||
! Vapor Pressure<br>(SI units) | |||
! Vapor Pressure<br>(Bar); | |||
! Vapor Pressure<br>(mmHg); | |||
! Temperature | |||
|- | |||
| [[Tungsten]] | |||
| 100 Pa | |||
| 0.001 | |||
| 0.75 | |||
| 3203 °C | |||
|- | |||
| [[Ethylene glycol]] | |||
| 500 Pa | |||
| 0.005 | |||
| 3.75 | |||
| 20 °C | |||
|- | |||
| [[Xenon difluoride]] | |||
| 600 Pa | |||
| 0.006 | |||
| 4.50 | |||
| 25 °C | |||
|- | |||
| [[Water]] (H<sub>2</sub>O) | |||
| 2.3 kPa | |||
| 0.023 | |||
| 17.5 | |||
| 20 °C | |||
|- | |||
| [[Propanol]] | |||
| 2.4 kPa | |||
| 0.024 | |||
| 18.0 | |||
| 20 °C | |||
|- | |||
| [[Ethanol]] | |||
| 5.83 kPa | |||
| 0.0583 | |||
| 43.7 | |||
| 20 °C | |||
|- | |||
| [[Methyl isobutyl ketone]] | |||
| 2.66 kPa | |||
| 0.0266 | |||
| 19.95 | |||
| 25 °C | |||
|- | |||
| [[Freon|Freon 113]] | |||
| 37.9 kPa | |||
| 0.379 | |||
| 284 | |||
| 20 °C | |||
|- | |||
| [[Acetaldehyde]] | |||
| 98.7 kPa | |||
| 0.987 | |||
| 740 | |||
| 20 °C | |||
|- | |||
| [[Butane]] | |||
| 220 kPa | |||
| 2.2 | |||
| 1650 | |||
| 20 °C | |||
|- | |||
| [[Formaldehyde]] | |||
| 435.7 kPa | |||
| 4.357 | |||
| 3268 | |||
| 20 °C | |||
|- | |||
| [[Propane]] | |||
| 1.013 MPa | |||
| 10.133 | |||
| 7600 | |||
| 25.6 °C | |||
|- | |||
| [[Carbonyl sulfide]] | |||
| 1.255 MPa | |||
| 12.55 | |||
| 9412 | |||
| 25 °C | |||
|- | |||
| [[Carbon dioxide]] | |||
| 5.7 MPa | |||
| 57 | |||
| 42753 | |||
| 20 °C | |||
|- | |||
|} | |||
==Estimating vapor pressure from molecular structure== | |||
Several empirical methods exist to estimate liquid vapor pressure from molecular structure for organic molecules. Some examples are SIMPOL,<ref>{{cite journal|author=J. F. Pankow et al.|title=SIMPOL.1: a simple group contribution method for predicting vapor pressures and enthalpies of vaporization of multifunctional organic compounds|journal=Atmos. Chem. Phys.|volume=8|pages=2773–2796|year=2008|url=http://www.atmos-chem-phys.net/8/2773/2008/acp-8-2773-2008.html}}</ref> the method of Moller et al.,<ref name = "Moller" /> and EVAPORATION.<ref>[http://tropo.aeronomie.be/models/evaporation_run.htm "Vapour pressure of pure liquid compounds. Estimation by EVAPORATION"]</ref><ref>{{cite journal|author=S. Compernolle et al.|title=EVAPORATION: a new vapour pressure estimation method for organic molecules including non-additivity and intramolecular interactions|journal=Atmos. Chem. Phys.|volume=11|pages=9431–9450|year=2011|url=http://www.atmos-chem-phys.net/11/9431/2011/acp-11-9431-2011.html}}</ref> | |||
==Meaning in meteorology== | |||
In [[meteorology]], the term ''vapor pressure'' is used to mean the [[partial pressure]] of [[water vapor]] in the atmosphere, even if it is not in equilibrium,<ref>[http://amsglossary.allenpress.com/glossary/search?id=vapor-pressure1 Glossary] (Developed by the [[American Meteorological Society]])</ref> and the ''equilibrium vapor pressure'' is specified otherwise. Meteorologists also use the term ''saturation vapor pressure'' to refer to the equilibrium vapor pressure of water or [[brine]] above a flat surface, to distinguish it from equilibrium vapor pressure, which takes into account the shape and size of water droplets and particulates in the atmosphere.<ref>[http://fermi.jhuapl.edu/people/babin/vapor/index.html A Brief Tutorial] (An article about the definition of equilibrium vapor pressure)</ref> | |||
==See also== | |||
* [[Absolute humidity]] | |||
* [[Clausius-Clapeyron relation]] | |||
* [[Partial pressure]] | |||
* [[Relative humidity]] | |||
* [[Relative volatility]] | |||
* [[Raoult's law]] | |||
* [[Saturation vapor density]] | |||
* [[Triple point]] | |||
* [[Vapor-liquid equilibrium]] | |||
* [[Vapour pressure of water|Vapor pressure of water]] | |||
* [[Volatility (chemistry)|Volatility]] | |||
* [[Reid vapor pressure]] | |||
* [[True vapor pressure]] | |||
* [[Vapor pressures of the elements (data page)]] | |||
==References== | |||
{{Reflist}} | |||
==External links== | |||
*[http://www.engineersedge.com/fluid_flow/fluid_data.htm Fluid Characteristics Chart] | |||
*[http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/vappre.html#c2 Hyperphysics] | |||
*[http://www.ilpi.com/msds/ref/vaporpressure.html MSDS Vapor Pressure] | |||
*[http://www.envmodels.com/freetools.php?menu=pression Online vapor pressure calculation tool (Requires Registration)] | |||
*[http://www.aim.env.uea.ac.uk/aim/ddbst/pcalc_main.php Prediction of Vapor Pressures of Pure Liquid Organic Compounds] | |||
[[Category:Thermodynamic properties]] | |||
[[Category:Chemical engineering]] | |||
[[Category:Meteorology]] | |||
[[Category:Gases]] | |||
[[Category:Pressure]] | |||
[[fr:Pression de vapeur saturante]] | |||
Revision as of 11:22, 31 January 2014

Vapor pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as volatile.
The vapor pressure of any substance increases non-linearly with temperature according to the Clausius–Clapeyron relation. The atmospheric pressure boiling point of a liquid (also known as the normal boiling point) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapor bubbles inside the bulk of the substance. Bubble formation deeper in the liquid requires a higher pressure, and therefore higher temperature, because the fluid pressure increases above the atmospheric pressure as the depth increases.
The vapor pressure that a single component in a mixture contributes to the total pressure in the system is called partial pressure. For example, air at sea level, and saturated with water vapor at 20 °C, has partial pressures of about 23 mbar of water, 780 mbar of nitrogen, 210 mbar of oxygen and 9 mbar of argon.
Measurement and units
Vapor pressure is measured in the standard units of pressure. The International System of Units (SI) recognizes pressure as a derived unit with the dimension of force per area and designates the pascal (Pa) as its standard unit. One pascal is one newton per square meter (N·m−2 or kg·m−1·s−2).
Experimental measurement of vapor pressure is a simple procedure for common pressures between 1 and 200 kPa.[1] Most accurate results are obtained near the boiling point of substances and large errors result for measurements smaller than Template:Gaps. Procedures often consist of purifying the test substance, isolating it in a container, evacuating any foreign gas, then measuring the equilibrium pressure of the gaseous phase of the substance in the container at different temperatures. Better accuracy is achieved when care is taken to ensure that the entire substance and its vapor are at the prescribed temperature. This is often done, as with the use of an isoteniscope, by submerging the containment area in a liquid bath.
Estimating vapor pressures with Antoine equation
The Antoine equation [2][3] is a mathematical expression of the relation between the vapor pressure and the temperature of pure liquid or solid substances. The basic form of the equation is:
and it can be transformed into this temperature-explicit form:
where: is the absolute vapor pressure of a substance
- is the temperature of the substance
- , and are substance-specific coefficients (i.e., constants or parameters)
- is typically either or [3]
A simpler form of the equation with only two coefficients is sometimes used:
which can be transformed to:
Sublimations and vaporizations of the same substance have separate sets of Antoine coefficients, as do components in mixtures.[2] Each parameter set for a specific compound is only applicable over a specified temperature range. Generally, temperature ranges are chosen to maintain the equation's accuracy of a few up to 8-10 percent. For many volatile substances, several different sets of parameters are available and used for different temperature ranges. The Antoine equation has poor accuracy with any single parameter set when used from a compound's melting point to its critical temperature. Accuracy is also usually poor when vapor pressure is under 10 Torr because of the limitations of the apparatus used to establish the Antoine parameter values.
The Wagner Equation[4] gives "one of the best"[5] fits to experimental data but is quite complex. It expresses reduced vapor pressure as a function of reduced temperature.
Relation to boiling point of liquids
47 year-old Podiatrist Hyslop from Alert Bay, has lots of hobbies and interests that include fencing, property developers in condo new launch singapore and handball. Just had a family trip to Monasteries of Haghpat and Sanahin.
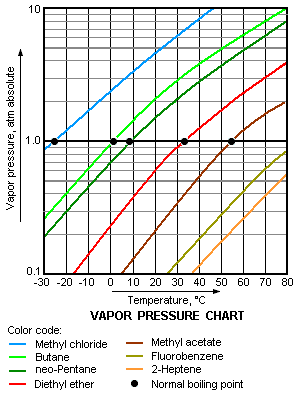
As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing boiling points. This is illustrated in the vapor pressure chart (see right) that shows graphs of the vapor pressures versus temperatures for a variety of liquids.[6]
For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. It also has the lowest normal boiling point (−24.2 °C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure.
Although the relation between vapor pressure and temperature is non-linear, the chart uses a logarithmic vertical axis to produce slightly curved lines, so one chart can graph many liquids. A nearly straight line is obtained when the logarithm of the vapor pressure is plotted against 1/(T+230)[7] where T is the temperature in degrees Celsius. The vapor pressure of a liquid at its boiling point equals the pressure of its surrounding environment.
Liquid mixtures
Raoult's law gives an approximation to the vapor pressure of mixtures of liquids. It states that the activity (pressure or fugacity) of a single-phase mixture is equal to the mole-fraction-weighted sum of the components' vapor pressures:
where p tot is the mixture's vapor pressure, i is one of the components of the mixture and Χi is the mole fraction of that component in the liquid mixture. The term piΧi is the partial pressure of component i in the mixture. Raoult's Law is applicable only to non-electrolytes (uncharged species); it is most appropriate for non-polar molecules with only weak intermolecular attractions (such as London forces).
Systems that have vapor pressures higher than indicated by the above formula are said to have positive deviations. Such a deviation suggests weaker intermolecular attraction than in the pure components, so that the molecules can be thought of as being "held in" the liquid phase less strongly than in the pure liquid. An example is the azeotrope of approximately 95% ethanol and water. Because the azeotrope's vapor pressure is higher than predicted by Raoult's law, it boils at a temperature below that of either pure component.
There are also systems with negative deviations that have vapor pressures that are lower than expected. Such a deviation is evidence for stronger intermolecular attraction between the constituents of the mixture than exists in the pure components. Thus, the molecules are "held in" the liquid more strongly when a second molecule is present. An example is a mixture of trichloromethane (chloroform) and 2-propanone (acetone), which boils above the boiling point of either pure component.
The negative and positive deviations can be used to determine thermodynamic activity coefficients of the components of mixtures.
Solids

Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in equilibrium with its own vapor. In the case of an equilibrium solid, such as a crystal, this can be defined as the pressure when the rate of sublimation of a solid matches the rate of deposition of its vapor phase. For most solids this pressure is very low, but some notable exceptions are naphthalene, dry ice (the vapor pressure of dry ice is 5.73 MPa (831 psi, 56.5 atm) at 20 degrees Celsius, which causes most sealed containers to rupture), and ice. All solid materials have a vapor pressure. However, due to their often extremely low values, measurement can be rather difficult. Typical techniques include the use of thermogravimetry and gas transpiration.
There are a number of methods for calculating the sublimation pressure (i.e., the vapor pressure) of a solid. One method is to estimate the sublimation pressure from extrapolated liquid vapor pressures (of the supercooled liquid), if the heat of fusion is known, by using this particular form of the Clausius–Clapeyron relation:[8]
with:
| = Sublimation pressure of the solid component at the temperature | |
| = Extrapolated vapor pressure of the liquid component at the temperature | |
| = Heat of fusion | |
| = Gas constant | |
| = Sublimation temperature | |
| = Melting point temperature |
This method assumes that the heat of fusion is temperature-independent, ignores additional transition temperatures between different solid phases, and it gives a fair estimation for temperatures not too far from the melting point. It also shows that the sublimation pressure is lower than the extrapolated liquid vapor pressure (ΔHm is positive) and the difference grows with increased distance from the melting point.
Boiling point of water

Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of water for atmospheric pressures can be approximated by the Antoine equation:
or transformed into this temperature-explicit form:
where the temperature is the boiling point in degrees Celsius and the pressure is in Torr.
Dühring's rule
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. Dühring's rule states that a linear relationship exists between the temperatures at which two solutions exert the same vapor pressure.
Examples
The following table is a list of a variety of substances ordered by increasing vapor pressure.
| Substance | Vapor Pressure (SI units) |
Vapor Pressure (Bar); |
Vapor Pressure (mmHg); |
Temperature |
|---|---|---|---|---|
| Tungsten | 100 Pa | 0.001 | 0.75 | 3203 °C |
| Ethylene glycol | 500 Pa | 0.005 | 3.75 | 20 °C |
| Xenon difluoride | 600 Pa | 0.006 | 4.50 | 25 °C |
| Water (H2O) | 2.3 kPa | 0.023 | 17.5 | 20 °C |
| Propanol | 2.4 kPa | 0.024 | 18.0 | 20 °C |
| Ethanol | 5.83 kPa | 0.0583 | 43.7 | 20 °C |
| Methyl isobutyl ketone | 2.66 kPa | 0.0266 | 19.95 | 25 °C |
| Freon 113 | 37.9 kPa | 0.379 | 284 | 20 °C |
| Acetaldehyde | 98.7 kPa | 0.987 | 740 | 20 °C |
| Butane | 220 kPa | 2.2 | 1650 | 20 °C |
| Formaldehyde | 435.7 kPa | 4.357 | 3268 | 20 °C |
| Propane | 1.013 MPa | 10.133 | 7600 | 25.6 °C |
| Carbonyl sulfide | 1.255 MPa | 12.55 | 9412 | 25 °C |
| Carbon dioxide | 5.7 MPa | 57 | 42753 | 20 °C |
Estimating vapor pressure from molecular structure
Several empirical methods exist to estimate liquid vapor pressure from molecular structure for organic molecules. Some examples are SIMPOL,[9] the method of Moller et al.,[8] and EVAPORATION.[10][11]
Meaning in meteorology
In meteorology, the term vapor pressure is used to mean the partial pressure of water vapor in the atmosphere, even if it is not in equilibrium,[12] and the equilibrium vapor pressure is specified otherwise. Meteorologists also use the term saturation vapor pressure to refer to the equilibrium vapor pressure of water or brine above a flat surface, to distinguish it from equilibrium vapor pressure, which takes into account the shape and size of water droplets and particulates in the atmosphere.[13]
See also
- Absolute humidity
- Clausius-Clapeyron relation
- Partial pressure
- Relative humidity
- Relative volatility
- Raoult's law
- Saturation vapor density
- Triple point
- Vapor-liquid equilibrium
- Vapor pressure of water
- Volatility
- Reid vapor pressure
- True vapor pressure
- Vapor pressures of the elements (data page)
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
External links
- Fluid Characteristics Chart
- Hyperphysics
- MSDS Vapor Pressure
- Online vapor pressure calculation tool (Requires Registration)
- Prediction of Vapor Pressures of Pure Liquid Organic Compounds
fr:Pression de vapeur saturante
- ↑ Template:Cite web
- ↑ 2.0 2.1 What is the Antoine Equation? (Chemistry Department, Frostburg State University, Maryland)
- ↑ 3.0 3.1 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ Many property agents need to declare for the PIC grant in Singapore. However, not all of them know find out how to do the correct process for getting this PIC scheme from the IRAS. There are a number of steps that you need to do before your software can be approved.
Naturally, you will have to pay a safety deposit and that is usually one month rent for annually of the settlement. That is the place your good religion deposit will likely be taken into account and will kind part or all of your security deposit. Anticipate to have a proportionate amount deducted out of your deposit if something is discovered to be damaged if you move out. It's best to you'll want to test the inventory drawn up by the owner, which can detail all objects in the property and their condition. If you happen to fail to notice any harm not already mentioned within the inventory before transferring in, you danger having to pay for it yourself.
In case you are in search of an actual estate or Singapore property agent on-line, you simply should belief your intuition. It's because you do not know which agent is nice and which agent will not be. Carry out research on several brokers by looking out the internet. As soon as if you end up positive that a selected agent is dependable and reliable, you can choose to utilize his partnerise in finding you a home in Singapore. Most of the time, a property agent is taken into account to be good if he or she locations the contact data on his website. This may mean that the agent does not mind you calling them and asking them any questions relating to new properties in singapore in Singapore. After chatting with them you too can see them in their office after taking an appointment.
Have handed an trade examination i.e Widespread Examination for House Brokers (CEHA) or Actual Property Agency (REA) examination, or equal; Exclusive brokers are extra keen to share listing information thus making certain the widest doable coverage inside the real estate community via Multiple Listings and Networking. Accepting a severe provide is simpler since your agent is totally conscious of all advertising activity related with your property. This reduces your having to check with a number of agents for some other offers. Price control is easily achieved. Paint work in good restore-discuss with your Property Marketing consultant if main works are still to be done. Softening in residential property prices proceed, led by 2.8 per cent decline within the index for Remainder of Central Region
Once you place down the one per cent choice price to carry down a non-public property, it's important to accept its situation as it is whenever you move in – faulty air-con, choked rest room and all. Get round this by asking your agent to incorporate a ultimate inspection clause within the possibility-to-buy letter. HDB flat patrons routinely take pleasure in this security net. "There's a ultimate inspection of the property two days before the completion of all HDB transactions. If the air-con is defective, you can request the seller to repair it," says Kelvin.
15.6.1 As the agent is an intermediary, generally, as soon as the principal and third party are introduced right into a contractual relationship, the agent drops out of the image, subject to any problems with remuneration or indemnification that he could have against the principal, and extra exceptionally, against the third occasion. Generally, agents are entitled to be indemnified for all liabilities reasonably incurred within the execution of the brokers´ authority.
To achieve the very best outcomes, you must be always updated on market situations, including past transaction information and reliable projections. You could review and examine comparable homes that are currently available in the market, especially these which have been sold or not bought up to now six months. You'll be able to see a pattern of such report by clicking here It's essential to defend yourself in opposition to unscrupulous patrons. They are often very skilled in using highly unethical and manipulative techniques to try and lure you into a lure. That you must also protect your self, your loved ones, and personal belongings as you'll be serving many strangers in your home. Sign a listing itemizing of all of the objects provided by the proprietor, together with their situation. HSR Prime Recruiter 2010 - ↑ Perry's Chemical Engineers' Handbook, 7th Ed. pg 4-15
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ Template:Cite article
- ↑ 8.0 8.1 Moller B., Rarey J., Ramjugernath D., "Estimation of the vapour pressure of non-electrolyte organic compounds via group contributions and group interactions ", J.Mol.Liq., 143(1), 52-63, 2008
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ "Vapour pressure of pure liquid compounds. Estimation by EVAPORATION"
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ Glossary (Developed by the American Meteorological Society)
- ↑ A Brief Tutorial (An article about the definition of equilibrium vapor pressure)























