Theoretical physics
Template:Pp-move-indef Template:Distinguish Template:Expert-subject

The atomic mass (ma) is the mass of an atomic particle, sub-atomic particle, or molecule. It may be expressed in unified atomic mass units; by international agreement, 1 atomic mass unit is defined as 1/12 of the mass of a single carbon-12 atom (at rest).[1] When expressed in such units, the atomic mass is called the relative isotopic mass (see section below).
The atomic mass or relative isotopic mass refers to the mass of a single particle, and is fundamentally different from the quantities elemental atomic weight (also called "relative atomic mass") and standard atomic weight, both of which refer to averages (mathematical means) of naturally-occurring atomic mass values for samples of elements. Such averages are expected to have a variance according to the sample source for the collection of nuclides that make up a sample of a chemical element (each of which has its own exact characteristic atomic mass). Such mixtures reflect various abundance ratios of isotopes of the element as the ratios naturally occur in the place where the element sample was collected. By contrast, atomic mass figures refer to identical particle species; due to the exactly identical nature of species of atomic particles, atomic mass values are expected to have no intrinsic variance at all. Atomic mass figures are thus commonly reported to many more significant figures than atomic weights.
The atomic mass of atoms, ions, or atomic nuclei is slightly less than the sum total mass of their constituting protons, neutrons, or electrons, due to binding energy mass loss (as per E=mc2).[2]
Relative isotopic mass: the same quantity as atomic mass, but with different units
Relative isotopic mass is not to be confused with the averaged quantity "relative atomic mass," which is the same as atomic weight (see above). Relative isotopic mass is similar to atomic mass and has exactly the same numerical value as atomic mass, whenever atomic mass is expressed in unified atomic mass units. However, relative isotopic mass is a pure number with no units. This loss of units results from the use of a scaling ratio with respect to a carbon-12 standard, and the word "relative" in the term "relative isotopic mass" refers to this scaling relative to carbon-12.
The relative isotopic mass, then, is the mass of a given isotope (specifically, any single nuclide), when this value is scaled by the mass of carbon-12, when the latter is set equal to 12. Equivalently, the relative isotopic mass of an isotope or nuclide is the mass of the isotope relative to 1/12 of the mass of a carbon-12 atom.
For example, the relative isotopic mass of carbon-12 is exactly 12, but (as in the case of atomic mass) no nuclides other than carbon-12 have exactly whole-number values in this scale. As is the case for the related atomic mass when expressed in unified atomic mass units, the relative isotopic mass numbers of nuclides other than carbon-12 are not whole numbers, but are always close to whole numbers. This is discussed more fully below.
Similar terms for different quantities
The atomic mass and relative isotopic mass are sometimes confused, or incorrectly used, as synonyms of relative atomic mass (also known as atomic weight) and the standard atomic weight (a particular variety of atomic weight). However, as noted in the introduction, atomic weight and standard atomic weight represent terms for averages of isotopic abundances, not for single nuclides. As such, atomic weight and standard atomic weight often differ numerically from relative isotopic mass, and they can also have different units than atomic mass when this quantity is not expressed in unified atomic mass units (see the linked article for atomic weight).
The atomic mass (relative isotopic mass) is defined as the mass of a single atom, which can only be one isotope (nuclide) at a time, and is not an abundance-weighted average, as in the case of relative atomic mass/atomic weight. The atomic mass or relative isotopic mass of each isotope and nuclide of a chemical element is therefore a number that can in principle be measured to a very great precision, since every specimen of such a nuclide is expected to be exactly identical to every other specimen, as all atoms of a given type in the same energy state, and every specimen of a particular nuclide, are expected to be exactly identical in mass to every other specimen of that nuclide. For example, every atom of oxygen-16 is expected to have exactly the same atomic mass (relative atomic mass) as every other atom of oxygen-16.
In the case of many elements that have one naturally occurring isotope (mononuclidic elements) or one dominant isotope, the actual numerical similarity/difference between the atomic mass of the most common isotope, and the relative atomic mass or (standard) atomic weight can be small or even nil, and does affect most bulk calculations. However, such an error can exist and even be important when considering individual atoms for elements that are not mononuclidic.
For non-mononuclidic elements that have more than one common isotope, the numerical difference in relative atomic mass (atomic weight) from even the most common relative isotopic mass, can be half a mass unit or more (e.g. see the case of chlorine where atomic weight and standard atomic weight are about 35.45). The atomic mass (relative isotopic mass) of an uncommon isotope can differ from the relative atomic mass, atomic weight, or standard atomic weight, by several mass units.
Atomic masses expressed in unified atomic mass units (i.e. relative isotopic masses) are always close to whole-number values, but never (except in the case of carbon-12) exactly a whole number. The difference from whole numbers for these values is due to two factors: [1] the different mass of neutrons and protons acting to change the total mass in nuclides that have a proton:neutron ratios other than the 1:1 ratio of carbon-12; and [2] an exact whole-number would not be expected if there exists a loss/gain of mass signifying a difference in mean binding energy relative to the mean binding energy for carbon-12. Experimentally, this is always the case.
The ratio of atomic mass to mass number varies from about 0.99884 for 56Fe to 1.00782505 for 1H.
Any mass defect due to nuclear binding energy is experimentally a small fraction (less than 1%) compared to the mass of a nucleon, and is an even smaller percentage compared to the average mass per nucleon in carbon-12, which is moderately strongly-bound compared with other atoms. Since protons and neutrons differ from each other in mass by an even smaller fraction (about 0.0014 u), the practice of rounding the atomic mass of any given nuclide or isotope to the nearest whole number, always gives the simple whole number total nucleon count, or mass number. The neutron count (neutron number) may then be derived by subtracting the number of protons (atomic number).
Mass defects in atomic masses
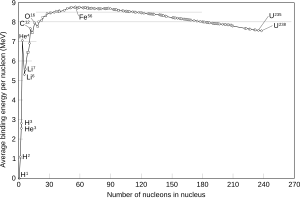
The amount that the ratio of atomic masses to mass number deviates from 1 is as follows: the deviation starts positive at hydrogen-1, becomes negative until a minimum is reached at iron-56, iron-58 and nickel-62, then increases to positive values in the heavy isotopes, with increasing atomic number. This corresponds to the fact that nuclear fission in an element heavier than zirconium produces energy, and fission in any element lighter than niobium requires energy. On the other hand, nuclear fusion reactions: fusion of two atoms of an element lighter than scandium produces energy, whereas fusion in elements heavier than calcium requires energy.
The difference mass number minus atomic mass is not maximized at iron-56 (where it is around 0.06 atomic mass units), but at heavier elements where it reaches about 0.10 atomic mass units.
Here are some values of the ratio of atomic mass to mass number:
| Nuclide | Ratio of atomic mass to mass number |
|---|---|
| 1H | 1.00782505 |
| 2H | 1.0070508885 |
| 3H | 1.0053497592 |
| 3He | 1.0053431064 |
| 4He | 1.0006508135 |
| 6Li | 1.0025204658 |
| 12C | 1 |
| 14N | 1.0002195718 |
| 16O | 0.9996821637 |
| 56Fe | 0.9988381696 |
| 210Po | 0.9999184462 |
| 232Th | 1.0001640315 |
| 238U | 1.0002133958 |
Measurement of atomic masses
Direct comparison and measurement of the masses of atoms is achieved with mass spectrometry.
Conversion factor between atomic mass units and grams
The standard scientific unit for dealing with atoms in macroscopic quantities is the mole (symbol: mol), which is defined arbitrarily as the amount of a substance with as many atoms or other units as there are in 12 grams of the carbon isotope C-12. The number of atoms in a mole is called Avogadro's number, the value of which is approximately 6.022 × 10Template:Smsup mol−1.
One mole of a substance always contains almost exactly the relative atomic mass or molar mass of that substance (which is the concept of molar mass), expressed in grams; however, this may or may not be true for the atomic mass, depending on whether or not the element exists naturally in more than one isotope. For example, the relative atomic mass of iron is 55.847 g/mol, and therefore one mole of iron as commonly found on earth has a mass of 55.847 grams. The atomic mass of the 56Fe isotope is 55.935 u and one mole of 56Fe atoms would then in theory weigh 55.935 g, but such amounts of pure 56Fe have never been found on Earth. However, there exist in nature 22 mononuclidic elements for which essentially only a single isotope is found in nature (common examples are fluorine, sodium, aluminum and phosphorus), and for these elements the relative atomic mass and atomic mass are the same. Samples of these elements therefore may serve as reference standards for certain atomic mass values.
The formulaic conversion between atomic mass units and SI mass in grams for a single atom is:
where is the Molar mass constant and is the Avogadro constant.
Relationship between atomic and molecular masses
Similar definitions apply to molecules. One can compute the molecular mass of a compound by adding the atomic masses of its constituent atoms (nuclides). One can compute the molar mass of a compound by adding the relative atomic masses of the elements given in the chemical formula. In both cases the multiplicity of the atoms (the number of times it occurs) must be taken into account, usually by multiplication of each unique mass by its multiplicity.
History
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. The first scientists to determine relative atomic masses were John Dalton and Thomas Thomson between 1803 and 1805 and Jöns Jakob Berzelius between 1808 and 1826. 'Relative atomic mass (Atomic weight) was originally defined relative to that of the lightest element hydrogen taken as 1.00, and in the 1820s Prout's hypothesis stated that atomic masses of all elements would prove via a whole number rule to be exact multiples of this hydrogen relative atomic mass. Berzelius, however, soon proved that this hypothesis did not always hold even approximately, and in some elements, such as chlorine, relative atomic mass falls almost exactly between two multiples of the hydrogen relative atomic mass. Still later, as noted, this was shown to be an isotope effect, and that the atomic masses of pure isotopes, or nuclides, are multiples of the hydrogen mass, to within about 1%.
In the 1860s Stanislao Cannizzaro refined relative atomic masses by applying Avogadro's law (notably at the Karlsruhe Congress of 1860). He formulated a law to determine relative atomic masses of elements: the different quantities of the same element contained in different molecules are all whole multiples of the atomic weight and determined relative atomic masses and molecular masses by comparing the vapor density of a collection of gases with molecules containing one or more of the chemical element in question.[3]
In the early twentieth century, up until the 1960s chemists and physicists used two different atomic-mass scales. The chemists used a scale such that the natural mixture of oxygen isotopes had an atomic mass 16, while the physicists assigned the same number 16 to the atomic mass of the most common oxygen isotope (containing eight protons and eight neutrons). However, because oxygen-17 and oxygen-18 are also present in natural oxygen this led to 2 different tables of atomic mass. The unified scale based on carbon-12, 12C, met the physicists' need to base the scale on a pure isotope, while being numerically close to the chemists' scale.
The term atomic weight is being phased out slowly and being replaced by relative atomic mass, in most current usage. The history of this shift in nomenclature reaches back to the 1960s and has been the source of much debate in the scientific community. The debate was largely created by the adoption of the unified atomic mass unit and the realization that weight was in some ways an inappropriate term. The argument for keeping the term "atomic weight" was primarily that it was a well understood term to those in the field, that the term "atomic mass" was already in use (as it is currently defined) and that the term "relative atomic mass" was in some ways redundant. In 1979, in a compromise move, the definition was refined and the term "relative atomic mass" was introduced as a secondary synonym. Twenty years later the primacy of these synonyms was reversed and the term "relative atomic mass" is now the preferred term; however the "standard atomic weights" have maintained the same name.[4]
See also
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
External links
- NIST relative atomic masses of all isotopes and the standard atomic weights of the elements
- Tutorial on the concept and measurement of atomic mass
- AME2003 Atomic Mass Evaluation from the National Nuclear Data Center
Real Estate Agent Renaldo Lester from Saint-Jean-Chrysostome, has several hobbies which include leathercrafting, property developers in singapore apartment for sale, this contact form, and crochet. Loves to see new cities and places like Ruins of Loropéni.
- ↑ Template:GoldBookRef
- ↑ Atomic mass, Encyclopædia Britannica on-line
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang


