Papyrus 24: Difference between revisions
en>Leszek Jańczuk |
en>Leszek Jańczuk |
||
| Line 1: | Line 1: | ||
{{Nuclear physics}} | |||
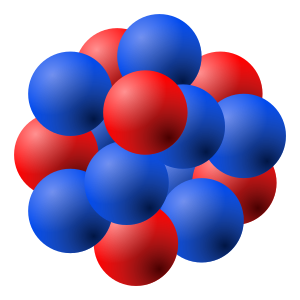
[[File:Nucleus drawing.svg|thumb|right|A model of the atomic nucleus showing it as a compact bundle of the two types of [[nucleon]]s: protons (red) and neutrons (blue). In this diagram, protons and neutrons look like little balls stuck together, but an actual nucleus (as understood by modern [[nuclear physics]]) cannot be explained like this, but only by using [[quantum mechanics]]. In a nucleus which occupies a certain [[energy level]] (for example, the [[ground state]]), each nucleon has multiple locations at once.]] | |||
The '''nucleus''' is the very dense region consisting of [[proton]]s and [[neutron]]s at the center of an [[atom]]. It was discovered in 1911 as a result of [[Ernest Rutherford]]'s interpretation of the 1909 [[Geiger–Marsden experiment|Geiger–Marsden gold foil experiment]] performed by [[Hans Geiger]] and [[Ernest Marsden]] under Rutherford's direction. The proton–neutron model of nucleus was proposed by [[Dmitry Ivanenko]] in 1932.<ref>{{cite book |author= Bernard Fernandez and Georges Ripka|title=Unravelling the Mystery of the Atomic Nucleus: A Sixty Year Journey 1896 — 1956 |chapter=Nuclear Theory After the Discovery of the Neutron |chapterurl=http://books.google.com/books?id=4PxRBakqFIUC&pg=PA263 |year=2012 |publisher=Springer |isbn=9781461441809 |page=263 |accessdate=15 February 2013}}</ref> Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the [[electron cloud]]. | |||
The diameter of the nucleus is in the range of {{val|1.75|ul=fm}} ({{val|1.75|e=-15|u=m}}) for [[hydrogen]] (the diameter of a single proton)<ref name=Nature> | |||
{{cite journal | |||
|author=Geoff Brumfiel | |||
|date=July 7, 2010 | |||
|title=The proton shrinks in size | |||
|journal=Nature | |||
|doi=10.1038/news.2010.337 | |||
}}</ref> to about {{val|15|u=fm}} for the heaviest atoms, such as [[uranium]]. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).{{cn|date=August 2013}} | |||
The branch of physics concerned with studying and understanding the atomic nucleus, including its composition and the forces which bind it together, is called [[nuclear physics]]. | |||
== Introduction == | |||
=== History === | |||
{{main|Rutherford model}} | |||
The nucleus was discovered in 1911, as a result of [[Ernest Rutherford]]'s efforts to test Thomson's "[[plum pudding model]]" of the atom.<ref>{{cite web | url=http://www.physics.rutgers.edu/meis/Rutherford.htm | title =The Rutherford Experiment | publisher =physics.rutgers.edu | author =''[[Rutgers University]]'' | accessdate =February 26, 2013}}</ref> The electron had already been discovered earlier by [[J.J. Thomson]] himself, and knowing that atoms are neutral, Thomson postulated that there must be a positive charge as well. In his plum pudding model, Thomson stated that an atom consisted of negative electrons randomly scattered within a sphere of positive charge. Ernest Rutherford later devised an experiment that involved the deflection of alpha particles at a thin sheet of metal foil. He reasoned that if Thomson's model were correct, the immense alpha particles would easily pass through the foil with very little deviation in their paths. To his surprise, many of the particles were deflected at very large angles. Because the mass of alpha particles is about 8000 times that of an electron, it became apparent that a very strong force was present that allowed the particles to be deflected. He realized that the plum pudding model could not be accurate and that the deflections of the alpha particles could only be caused by a center of concentrated charge that contained most of the atom's mass. Thus, the idea of a nuclear atom—an atom with a dense center of positive charge—became justified. | |||
===Etymology=== | |||
The term '''nucleus''' is from the Latin word ''nucleus'', a diminutive of [[wikt:nux|''nux'']] ("nut"), meaning the kernel (i.e., the "small nut") inside a watery type of fruit (like a peach). In 1844, [[Michael Faraday]] used the term to refer to the "central point of an atom". The modern atomic meaning was proposed by Ernest Rutherford in 1912.<ref> | |||
{{cite web | |||
|author=D. Harper | |||
|title=Nucleus | |||
|url=http://www.etymonline.com/index.php?search=Nucleus&searchmode=none | |||
|work=Online Etymology Dictionary | |||
|accessdate=2010-03-06 | |||
}}</ref> The adoption of the term "nucleus" to atomic theory, however, was not immediate. In 1916, for example, [[Gilbert N. Lewis]] stated, in his famous article ''The Atom and the Molecule'', that "the atom is composed of the ''kernel'' and an outer atom or ''shell''"<ref> | |||
{{cite journal | |||
|author=G.N. Lewis | |||
|year=1916 | |||
|title=The Atom and the Molecule | |||
|url=http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/papers/corr216.3-lewispub-19160400.html | |||
|journal=[[Journal of the American Chemical Society]] | |||
|volume=38 |page=4 | |||
|doi=10.1021/ja02261a002 | |||
|issue=4 | |||
}}</ref> | |||
=== Nuclear makeup === | |||
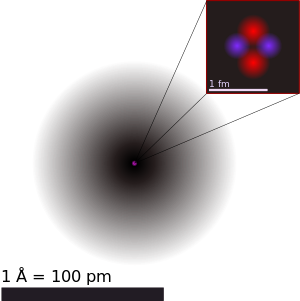
[[File:Helium atom QM.svg|right|300px|thumbnail|A figurative depiction of the [[helium]]-4 atom with the electron cloud in shades of gray. In the nucleus, the two protons and two neutrons are depicted in red and blue. This depiction shows the particles as separate, whereas in an actual helium atom, the protons are superimposed in space and most likely found at the very center of the nucleus, and the same is true of the two neutrons. Thus, all four particles are most likely found in exactly the same space, at the central point. Classical images of separate particles fail to model known charge distributions in very small nuclei. A more accurate image is that the spatial distribution of nucleons in helium's nucleus, although on a far smaller scale, is much closer to the helium '''electron cloud''' shown here, than to the fanciful nucleus image.]] | |||
The nucleus of an atom consists of protons and neutrons (two types of [[baryon]]s) bound by the [[nuclear force]] (also known as the '''residual strong force'''). These baryons are further composed of subatomic fundamental particles known as [[quark]]s bound by the [[strong interaction]]. Which [[chemical element]] an atom represents is determined by the number of [[proton]]s in the nucleus. Each proton carries a single [[electric charge|positive charge]], and the total electrical charge of the nucleus is spread fairly uniformly throughout its body, with a fall-off at the edge. | |||
Major exceptions to this rule are the light elements hydrogen and helium, where the charge is concentrated most highly at the single central point (without a central volume of uniform charge). This configuration is the same as for '''1s''' electrons in [[atomic orbital]]s, and is the expected density distribution for fermions (in this case, protons) in '''1s''' states without orbital angular momentum.<ref name=Basdevant> | |||
{{cite book | |||
|author=J.-L. Basdevant, J. Rich, M. Spiro | |||
|year=2005 | |||
|title=Fundamentals in Nuclear Physics | |||
|page=13, Fig. 1.1 | |||
|url=http://books.google.com/?id=OFx7P9mgC9oC&pg=PA375&dq=helium+%22nuclear+structure%22 | |||
|publisher=[[Springer (publisher)|Springer]] | |||
|isbn=0-387-01672-4 | |||
}}</ref> | |||
As each proton carries a unit of charge, the charge distribution is indicative of the proton distribution. The neutron distribution probably is similar.<ref name=Basdevant/> | |||
While protons define the entire charge of a nucleus and, hence, its [[chemical element|chemical identity]], neutrons are electrically neutral, but contribute to the mass of a nucleus to the same extent. Neutrons explain the phenomenon of [[isotope]]s – varieties of a chemical element which differ in [[atomic mass]]. | |||
==Protons and neutrons== | |||
Protons and neutrons are [[fermion]]s, with different values of the [[strong isospin]] [[quantum number]], so two protons and two neutrons can share the same space [[wave function]] since they are not identical quantum entities. They sometimes are viewed as two different quantum states of the same particle, the ''[[nucleon]]''.<ref name=Sitenko> | |||
{{cite book | |||
|author=A.G. Sitenko, V.K. Tartakovskiĭ | |||
|year=1997 | |||
|title=Theory of Nucleus: Nuclear Structure and Nuclear Interaction | |||
|url=http://books.google.com/?id=swb9QpqOqtAC&pg=PA464&dq=isbn=0-7923-4423-5#PPA3,M1 | |||
|page=3 | |||
|publisher=[[Kluwer Academic]] | |||
|isbn=0-7923-4423-5 | |||
}}</ref><ref name=Srednicki> | |||
{{cite book | |||
|author=M.A. Srednicki | |||
|year=2007 | |||
|title=Quantum Field Theory | |||
|pages=522–523 | |||
|publisher=[[Cambridge University Press]] | |||
|isbn=978-0-521-86449-7 | |||
}}</ref> Two fermions, such as two protons, or two neutrons, or a proton + neutron (the deuteron) can exhibit bosonic behavior when they become loosely bound in pairs. | |||
In the rare case of a [[hypernucleus]], a third [[baryon]] called a [[hyperon]], with a different value of the [[strangeness]] quantum number can also share the wave function. However, the latter type of nuclei are extremely unstable and are not found on Earth except in high energy physics experiments. | |||
The neutron has a positively charged core of radius ≈ 0.3 fm surrounded by a compensating negative charge of radius between 0.3 fm and 2 fm. The proton has an approximately exponentially decaying positive charge distribution with a mean square radius of about 0.8 fm.<ref name=Basdevant2> | |||
{{cite book | |||
|author=J.-L. Basdevant, J. Rich, M. Spiro | |||
|year=2005 | |||
|title=Fundamentals in Nuclear Physics | |||
|page=155 | |||
|url=http://books.google.com/?id=OFx7P9mgC9oC&pg=PA375&dq=helium+%22nuclear+structure%22 | |||
|publisher=[[Springer (publisher)|Springer]] | |||
|isbn=0-387-01672-4 | |||
}}</ref> | |||
==Forces== | |||
Nuclei are bound together by the residual strong force ([[nuclear force]]). The residual strong force is a minor residuum of the [[strong interaction]] which binds quarks together to form protons and neutrons. This force is much weaker ''between'' neutrons and protons because it is mostly neutralized within them, in the same way that electromagnetic forces ''between'' neutral atoms (such as [[van der Waals force]]s that act between two inert gas atoms) are much weaker than the electromagnetic forces that hold the parts of the atoms internally together (for example, the forces that hold the electrons in an inert gas atom bound to its nucleus). | |||
The nuclear force is highly attractive at the distance of typical nucleon separation, and this overwhelms the repulsion between protons which is due to the electromagnetic force, thus allowing nuclei to exist. However, because the residual strong force has a limited range because it decays quickly with distance (see [[Yukawa potential]]), only nuclei smaller than a certain size can be completely stable. The largest known completely stable (e.g., stable to alpha, beta, and gamma decay) nucleus is [[lead-208]] which contains a total of 208 nucleons (126 neutrons and 82 protons). Nuclei larger than this maximal size of 208 particles are unstable and (as a trend) become increasingly short-lived with larger size, as the number of neutrons and protons which compose them increases beyond this number. However, [[bismuth-209]] is also stable to beta decay and has the longest half-life to alpha decay of any known isotope, estimated at a billion times longer than the age of the universe. | |||
The residual strong force is effective over a very short range (usually only a few [[fermi (length)|fermis]]; roughly one or two nucleon diameters) and causes an attraction between any pair of nucleons. For example, between [[proton]]s and [[neutron]]s to form [NP] [[deuteron]], and also between protons and protons, and neutrons and neutrons. | |||
==Halo nuclei and strong force range limits== | |||
The effective absolute limit of the range of the strong force is represented by [[halo nucleus|halo nuclei]] such as [[lithium-11]] or [[boron-14]], in which [[dineutron]]s, or other collections of neutrons, orbit at distances of about ten fermis (roughly similar to the 8 fermi radius of the nucleus of uranium-238). These nuclei are not maximally dense. Halo nuclei form at the extreme edges of the chart of the nuclides—the neutron drip line and proton drip line—and are all unstable with short half-lives, measured in [[millisecond]]s; for example, lithium-11 has a half-life of less than 8.6 milliseconds. | |||
Halos in effect represent an excited state with nucleons in an outer quantum shell which has unfilled energy levels "below" it (both in terms of radius and energy). The halo may be made of either neutrons [NN, NNN] or protons [PP, PPP]. Nuclei which have a single neutron halo include <sup>11</sup>Be and <sup>19</sup>C. A two-neutron halo is exhibited by <sup>6</sup>He, <sup>11</sup>Li, <sup>17</sup>B, <sup>19</sup>B and <sup>22</sup>C. Two-neutron halo nuclei break into three fragments, never two, and are called ''[[Borromean nucleus|Borromean nuclei]]'' because of this behavior (referring to a system of three interlocked rings in which breaking any ring frees both of the others). <sup>8</sup>He and <sup>14</sup>Be both exhibit a four-neutron halo. Nuclei which have a proton halo include <sup>8</sup>B and <sup>26</sup>P. A two-proton halo is exhibited by <sup>17</sup>Ne and <sup>27</sup>S. Proton halos are expected to be more rare and unstable than the neutron examples, because of the repulsive electromagnetic forces of the excess proton(s). | |||
==Nuclear models== | |||
Although the [[standard model]] of physics is widely believed to completely describe the composition and behavior of the nucleus, generating predictions from theory is much more difficult than for most other areas of [[particle physics]]. This is essentially because [[Perturbation_theory_(quantum_mechanics)|perturbation theory]], a widely used mathematical tool, is not applicable to [[quantum chromodynamics]] (the theory of the [[strong force]]) at the energy scales relevant to the nucleus. As a result, experiments have historically been compared to relatively crude models which are necessarily imperfect. None of these models completely explain experimental data on nuclear structure.<ref name=Cook> | |||
{{cite book | |||
|author=N.D. Cook | |||
|year=2010 | |||
|title=Models of the Atomic Nucleus | |||
|edition=2nd | |||
|page=57 ff. | |||
|publisher=[[Springer Science+Business Media|Springer]] | |||
|isbn=978-3-642-14736-4 | |||
}}</ref> | |||
The [[nuclear size|nuclear radius]] (''R'') is considered to be one of the basic quantities that any model must predict. For stable nuclei (not halo nuclei or other unstable distorted nuclei) the nuclear radius is roughly proportional to the cube root of the [[mass number]] (''A'') of the nucleus, and particularly in nuclei containing many nucleons, as they arrange in more spherical configurations: | |||
The stable nucleus has approximately a constant density and therefore the nuclear radius R can be approximated by the following formula, | |||
:<math>R = r_0 A^{1/3} \,</math> | |||
where ''A'' = Atomic [[mass number]] (the number of protons ''Z'', plus the number of neutrons ''N'') and ''r''<sub>0</sub> = 1.25 fm = 1.25 × 10<sup>−15</sup> m. In this equation, the constant ''r''<sub>0</sub> varies by 0.2 fm, depending on the nucleus in question, but this is less than 20% change from a constant.<ref> | |||
{{cite book | |||
|author=K.S. Krane | |||
|year=1987 | |||
|title=Introductory Nuclear Physics | |||
|page= | |||
|publisher=[[John Wiley & Sons|Wiley-VCH]] | |||
|isbn=0-471-80553-X | |||
}}</ref> | |||
In other words, packing protons and neutrons in the nucleus gives ''approximately'' the same total size result as packing hard spheres of a constant size (like marbles) into a tight spherical or almost spherical bag (some stable nuclei are not quite spherical, but are known to be [[prolate]]).{{citation needed|date=December 2010}} | |||
=== Liquid drop models === | |||
{{Main|Liquid-drop model}} | |||
Early models of the nucleus viewed the nucleus as a rotating liquid drop. In this model, the trade-off of long-range electromagnetic forces and relatively short-range nuclear forces, together cause behavior which resembled surface tension forces in liquid drops of different sizes. This formula is successful at explaining many important phenomena of nuclei, such as their changing amounts of [[binding energy]] as their size and composition changes (see [[semi-empirical mass formula]]), but it does not explain the special stability which occurs when nuclei have special "magic numbers" of protons or neutrons. | |||
=== Shell models and other quantum models === | |||
{{Main| Nuclear shell model}} | |||
A number of models for the nucleus have also been proposed in which nucleons occupy orbitals, much like the [[atomic orbital]]s in [[atomic physics]] theory. These wave models imagine nucleons to be either sizeless point particles in potential wells, or else probability waves as in the "optical model", frictionlessly orbiting at high speed in potential wells. | |||
In the above models, the nucleons may occupy orbitals in pairs, due to being fermions, which allows to explain [[even and odd atomic nuclei|even/odd ''Z'' and ''N'' effects]] well-known from experiments. The exact nature and capacity of nuclear shells differs from those of electrons in atomic orbitals, primarily because the potential well in which the nucleons move (especially in larger nuclei) is quite different from the central electromagnetic potential well which binds electrons in atoms. Some resemblance to atomic orbital models may be seen in a small atomic nucleus like that of [[helium-4]], in which the two protons and two neutrons separately occupy 1s orbitals analogous to the 1s orbital for the two electrons in the helium atom, and achieve unusual stability for the same reason. Nuclei with 5 nucleons are all extremely unstable and short-lived, yet, [[helium-3]], with 3 nucleons, is very stable even with lack of a closed 1s orbital shell. Another nucleus with 3 nucleons, the triton [[hydrogen-3]] is unstable and will decay into helium-3 when isolated. Weak nuclear stability with 2 nucleons {NP} in the 1s orbital is found in the deuteron [[hydrogen-2]], with only one nucleon in each of the proton and neutron potential wells. While each nucleon is a fermion, the {NP} deuteron is a boson and thus does not follow Pauli Exclusion for close packing within shells. [[Lithium-6]] with 6 nucleons is highly stable without a closed second 1p shell orbital. For light nuclei with total nucleon numbers 1 to 6 only those with 5 do not show some evidence of stability. Observations of beta-stability of light nuclei outside closed shells indicate that nuclear stability is much more complex than simple closure of shell orbitals with [[magic number (physics)|magic numbers]] of protons and neutrons. | |||
For larger nuclei, the shells occupied by nucleons begin to differ significantly from electron shells, but nevertheless, present nuclear theory does predict the [[magic number (physics)|magic numbers]] of filled nuclear shells for both protons and neutrons. The closure of the stable shells predicts unusually stable configurations, analogous to the noble group of nearly-inert gases in chemistry. An example is the stability of the closed shell of 50 protons, which allows [[tin]] to have 10 stable isotopes, more than any other element. Similarly, the distance from shell-closure explains the unusual instability of isotopes which have far from stable numbers of these particles, such as the radioactive elements 43 ([[technetium]]) and 61 ([[promethium]]), each of which is preceded and followed by 17 or more stable elements. | |||
There are however problems with the shell model when an attempt is made to account for nuclear properties well away from closed shells. This has led to complex ''post hoc'' distortions of the shape of the potential well to fit experimental data, but the question remains whether these mathematical manipulations actually correspond to the spatial deformations in real nuclei. Problems with the shell model have led some to propose realistic two-body and three-body nuclear force effects involving nucleon clusters and then build the nucleus on this basis. Two such cluster models are the Close-Packed Spheron Model of Linus Pauling and the 2D Ising Model of MacGregor.<ref name=Cook/> | |||
=== Consistency between models === | |||
{{Main|Nuclear structure}} | |||
As with the case of [[superfluid]] [[liquid helium]], atomic nuclei are an example of a state in which both (1) "ordinary" particle physical rules for volume and (2) non-intuitive quantum mechanical rules for a wave-like nature apply. In superfluid helium, the helium atoms have volume, and essentially "touch" each other, yet at the same time exhibit strange bulk properties, consistent with a [[Bose–Einstein condensation]]. The latter reveals that they also have a wave-like nature and do not exhibit standard fluid properties, such as friction. For nuclei made of [[hadron]]s which are [[fermion]]s, the same type of condensation does not occur, yet nevertheless, many nuclear properties can only be explained similarly by a combination of properties of particles with volume, in addition to the frictionless motion characteristic of the wave-like behavior of objects trapped in [[Erwin Schrödinger]]'s [[Atomic orbital#Formal quantum mechanical definition|quantum orbital]]s. | |||
== See also == | |||
{{columns-list|2| | |||
*[[Giant resonance]] | |||
*[[List of particles]] | |||
*[[Nuclear medicine]] | |||
*[[Radioactivity]] | |||
*[[Semi-empirical mass formula]] | |||
}} | |||
== References == | |||
{{Reflist|35em}} | |||
== External links == | |||
* [http://www.lightandmatter.com/html_books/4em/ch02/ch02.html The Nucleus – a chapter from an online textbook] | |||
* [http://www-nds.iaea.org/livechart The LIVEChart of Nuclides – IAEA] in [http://www-nds.iaea.org/livechart Java ] or [http://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html HTML] | |||
* [http://www.halexandria.org/dward472.htm Article on the "nuclear shell model," giving nuclear shell filling for the various elements]. Accessed Sept. 16, 2009. | |||
* [http://nagysandor.eu/nuklearis/timeline/index.html Timeline: Subatomic Concepts, Nuclear Science & Technology]. | |||
{{particles}} | |||
{{Nuclear Technology}} | |||
{{DEFAULTSORT:Atomic Nucleus}} | |||
[[Category:Nuclear chemistry]] | |||
[[Category:Nuclear physics]] | |||
[[Category:Subatomic particles]] | |||
[[Category:Radiochemistry]] | |||
{{Link GA|uk}} | |||
Revision as of 23:29, 26 February 2013
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911 as a result of Ernest Rutherford's interpretation of the 1909 Geiger–Marsden gold foil experiment performed by Hans Geiger and Ernest Marsden under Rutherford's direction. The proton–neutron model of nucleus was proposed by Dmitry Ivanenko in 1932.[1] Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud.
The diameter of the nucleus is in the range of Template:Val (Template:Val) for hydrogen (the diameter of a single proton)[2] to about Template:Val for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).Template:Cn
The branch of physics concerned with studying and understanding the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.
Introduction
History
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. The nucleus was discovered in 1911, as a result of Ernest Rutherford's efforts to test Thomson's "plum pudding model" of the atom.[3] The electron had already been discovered earlier by J.J. Thomson himself, and knowing that atoms are neutral, Thomson postulated that there must be a positive charge as well. In his plum pudding model, Thomson stated that an atom consisted of negative electrons randomly scattered within a sphere of positive charge. Ernest Rutherford later devised an experiment that involved the deflection of alpha particles at a thin sheet of metal foil. He reasoned that if Thomson's model were correct, the immense alpha particles would easily pass through the foil with very little deviation in their paths. To his surprise, many of the particles were deflected at very large angles. Because the mass of alpha particles is about 8000 times that of an electron, it became apparent that a very strong force was present that allowed the particles to be deflected. He realized that the plum pudding model could not be accurate and that the deflections of the alpha particles could only be caused by a center of concentrated charge that contained most of the atom's mass. Thus, the idea of a nuclear atom—an atom with a dense center of positive charge—became justified.
Etymology
The term nucleus is from the Latin word nucleus, a diminutive of nux ("nut"), meaning the kernel (i.e., the "small nut") inside a watery type of fruit (like a peach). In 1844, Michael Faraday used the term to refer to the "central point of an atom". The modern atomic meaning was proposed by Ernest Rutherford in 1912.[4] The adoption of the term "nucleus" to atomic theory, however, was not immediate. In 1916, for example, Gilbert N. Lewis stated, in his famous article The Atom and the Molecule, that "the atom is composed of the kernel and an outer atom or shell"[5]
Nuclear makeup
The nucleus of an atom consists of protons and neutrons (two types of baryons) bound by the nuclear force (also known as the residual strong force). These baryons are further composed of subatomic fundamental particles known as quarks bound by the strong interaction. Which chemical element an atom represents is determined by the number of protons in the nucleus. Each proton carries a single positive charge, and the total electrical charge of the nucleus is spread fairly uniformly throughout its body, with a fall-off at the edge.
Major exceptions to this rule are the light elements hydrogen and helium, where the charge is concentrated most highly at the single central point (without a central volume of uniform charge). This configuration is the same as for 1s electrons in atomic orbitals, and is the expected density distribution for fermions (in this case, protons) in 1s states without orbital angular momentum.[6]
As each proton carries a unit of charge, the charge distribution is indicative of the proton distribution. The neutron distribution probably is similar.[6]
While protons define the entire charge of a nucleus and, hence, its chemical identity, neutrons are electrically neutral, but contribute to the mass of a nucleus to the same extent. Neutrons explain the phenomenon of isotopes – varieties of a chemical element which differ in atomic mass.
Protons and neutrons
Protons and neutrons are fermions, with different values of the strong isospin quantum number, so two protons and two neutrons can share the same space wave function since they are not identical quantum entities. They sometimes are viewed as two different quantum states of the same particle, the nucleon.[7][8] Two fermions, such as two protons, or two neutrons, or a proton + neutron (the deuteron) can exhibit bosonic behavior when they become loosely bound in pairs.
In the rare case of a hypernucleus, a third baryon called a hyperon, with a different value of the strangeness quantum number can also share the wave function. However, the latter type of nuclei are extremely unstable and are not found on Earth except in high energy physics experiments.
The neutron has a positively charged core of radius ≈ 0.3 fm surrounded by a compensating negative charge of radius between 0.3 fm and 2 fm. The proton has an approximately exponentially decaying positive charge distribution with a mean square radius of about 0.8 fm.[9]
Forces
Nuclei are bound together by the residual strong force (nuclear force). The residual strong force is a minor residuum of the strong interaction which binds quarks together to form protons and neutrons. This force is much weaker between neutrons and protons because it is mostly neutralized within them, in the same way that electromagnetic forces between neutral atoms (such as van der Waals forces that act between two inert gas atoms) are much weaker than the electromagnetic forces that hold the parts of the atoms internally together (for example, the forces that hold the electrons in an inert gas atom bound to its nucleus).
The nuclear force is highly attractive at the distance of typical nucleon separation, and this overwhelms the repulsion between protons which is due to the electromagnetic force, thus allowing nuclei to exist. However, because the residual strong force has a limited range because it decays quickly with distance (see Yukawa potential), only nuclei smaller than a certain size can be completely stable. The largest known completely stable (e.g., stable to alpha, beta, and gamma decay) nucleus is lead-208 which contains a total of 208 nucleons (126 neutrons and 82 protons). Nuclei larger than this maximal size of 208 particles are unstable and (as a trend) become increasingly short-lived with larger size, as the number of neutrons and protons which compose them increases beyond this number. However, bismuth-209 is also stable to beta decay and has the longest half-life to alpha decay of any known isotope, estimated at a billion times longer than the age of the universe.
The residual strong force is effective over a very short range (usually only a few fermis; roughly one or two nucleon diameters) and causes an attraction between any pair of nucleons. For example, between protons and neutrons to form [NP] deuteron, and also between protons and protons, and neutrons and neutrons.
Halo nuclei and strong force range limits
The effective absolute limit of the range of the strong force is represented by halo nuclei such as lithium-11 or boron-14, in which dineutrons, or other collections of neutrons, orbit at distances of about ten fermis (roughly similar to the 8 fermi radius of the nucleus of uranium-238). These nuclei are not maximally dense. Halo nuclei form at the extreme edges of the chart of the nuclides—the neutron drip line and proton drip line—and are all unstable with short half-lives, measured in milliseconds; for example, lithium-11 has a half-life of less than 8.6 milliseconds.
Halos in effect represent an excited state with nucleons in an outer quantum shell which has unfilled energy levels "below" it (both in terms of radius and energy). The halo may be made of either neutrons [NN, NNN] or protons [PP, PPP]. Nuclei which have a single neutron halo include 11Be and 19C. A two-neutron halo is exhibited by 6He, 11Li, 17B, 19B and 22C. Two-neutron halo nuclei break into three fragments, never two, and are called Borromean nuclei because of this behavior (referring to a system of three interlocked rings in which breaking any ring frees both of the others). 8He and 14Be both exhibit a four-neutron halo. Nuclei which have a proton halo include 8B and 26P. A two-proton halo is exhibited by 17Ne and 27S. Proton halos are expected to be more rare and unstable than the neutron examples, because of the repulsive electromagnetic forces of the excess proton(s).
Nuclear models
Although the standard model of physics is widely believed to completely describe the composition and behavior of the nucleus, generating predictions from theory is much more difficult than for most other areas of particle physics. This is essentially because perturbation theory, a widely used mathematical tool, is not applicable to quantum chromodynamics (the theory of the strong force) at the energy scales relevant to the nucleus. As a result, experiments have historically been compared to relatively crude models which are necessarily imperfect. None of these models completely explain experimental data on nuclear structure.[10]
The nuclear radius (R) is considered to be one of the basic quantities that any model must predict. For stable nuclei (not halo nuclei or other unstable distorted nuclei) the nuclear radius is roughly proportional to the cube root of the mass number (A) of the nucleus, and particularly in nuclei containing many nucleons, as they arrange in more spherical configurations:
The stable nucleus has approximately a constant density and therefore the nuclear radius R can be approximated by the following formula,
where A = Atomic mass number (the number of protons Z, plus the number of neutrons N) and r0 = 1.25 fm = 1.25 × 10−15 m. In this equation, the constant r0 varies by 0.2 fm, depending on the nucleus in question, but this is less than 20% change from a constant.[11]
In other words, packing protons and neutrons in the nucleus gives approximately the same total size result as packing hard spheres of a constant size (like marbles) into a tight spherical or almost spherical bag (some stable nuclei are not quite spherical, but are known to be prolate).Potter or Ceramic Artist Truman Bedell from Rexton, has interests which include ceramics, best property developers in singapore developers in singapore and scrabble. Was especially enthused after visiting Alejandro de Humboldt National Park.
Liquid drop models
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church.
Early models of the nucleus viewed the nucleus as a rotating liquid drop. In this model, the trade-off of long-range electromagnetic forces and relatively short-range nuclear forces, together cause behavior which resembled surface tension forces in liquid drops of different sizes. This formula is successful at explaining many important phenomena of nuclei, such as their changing amounts of binding energy as their size and composition changes (see semi-empirical mass formula), but it does not explain the special stability which occurs when nuclei have special "magic numbers" of protons or neutrons.
Shell models and other quantum models
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church.
A number of models for the nucleus have also been proposed in which nucleons occupy orbitals, much like the atomic orbitals in atomic physics theory. These wave models imagine nucleons to be either sizeless point particles in potential wells, or else probability waves as in the "optical model", frictionlessly orbiting at high speed in potential wells.
In the above models, the nucleons may occupy orbitals in pairs, due to being fermions, which allows to explain even/odd Z and N effects well-known from experiments. The exact nature and capacity of nuclear shells differs from those of electrons in atomic orbitals, primarily because the potential well in which the nucleons move (especially in larger nuclei) is quite different from the central electromagnetic potential well which binds electrons in atoms. Some resemblance to atomic orbital models may be seen in a small atomic nucleus like that of helium-4, in which the two protons and two neutrons separately occupy 1s orbitals analogous to the 1s orbital for the two electrons in the helium atom, and achieve unusual stability for the same reason. Nuclei with 5 nucleons are all extremely unstable and short-lived, yet, helium-3, with 3 nucleons, is very stable even with lack of a closed 1s orbital shell. Another nucleus with 3 nucleons, the triton hydrogen-3 is unstable and will decay into helium-3 when isolated. Weak nuclear stability with 2 nucleons {NP} in the 1s orbital is found in the deuteron hydrogen-2, with only one nucleon in each of the proton and neutron potential wells. While each nucleon is a fermion, the {NP} deuteron is a boson and thus does not follow Pauli Exclusion for close packing within shells. Lithium-6 with 6 nucleons is highly stable without a closed second 1p shell orbital. For light nuclei with total nucleon numbers 1 to 6 only those with 5 do not show some evidence of stability. Observations of beta-stability of light nuclei outside closed shells indicate that nuclear stability is much more complex than simple closure of shell orbitals with magic numbers of protons and neutrons.
For larger nuclei, the shells occupied by nucleons begin to differ significantly from electron shells, but nevertheless, present nuclear theory does predict the magic numbers of filled nuclear shells for both protons and neutrons. The closure of the stable shells predicts unusually stable configurations, analogous to the noble group of nearly-inert gases in chemistry. An example is the stability of the closed shell of 50 protons, which allows tin to have 10 stable isotopes, more than any other element. Similarly, the distance from shell-closure explains the unusual instability of isotopes which have far from stable numbers of these particles, such as the radioactive elements 43 (technetium) and 61 (promethium), each of which is preceded and followed by 17 or more stable elements.
There are however problems with the shell model when an attempt is made to account for nuclear properties well away from closed shells. This has led to complex post hoc distortions of the shape of the potential well to fit experimental data, but the question remains whether these mathematical manipulations actually correspond to the spatial deformations in real nuclei. Problems with the shell model have led some to propose realistic two-body and three-body nuclear force effects involving nucleon clusters and then build the nucleus on this basis. Two such cluster models are the Close-Packed Spheron Model of Linus Pauling and the 2D Ising Model of MacGregor.[10]
Consistency between models
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. As with the case of superfluid liquid helium, atomic nuclei are an example of a state in which both (1) "ordinary" particle physical rules for volume and (2) non-intuitive quantum mechanical rules for a wave-like nature apply. In superfluid helium, the helium atoms have volume, and essentially "touch" each other, yet at the same time exhibit strange bulk properties, consistent with a Bose–Einstein condensation. The latter reveals that they also have a wave-like nature and do not exhibit standard fluid properties, such as friction. For nuclei made of hadrons which are fermions, the same type of condensation does not occur, yet nevertheless, many nuclear properties can only be explained similarly by a combination of properties of particles with volume, in addition to the frictionless motion characteristic of the wave-like behavior of objects trapped in Erwin Schrödinger's quantum orbitals.
See also
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
External links
- The Nucleus – a chapter from an online textbook
- The LIVEChart of Nuclides – IAEA in Java or HTML
- Article on the "nuclear shell model," giving nuclear shell filling for the various elements. Accessed Sept. 16, 2009.
- Timeline: Subatomic Concepts, Nuclear Science & Technology.
Template:Particles Template:Nuclear Technology Template:Link GA
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑
One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ Template:Cite web
- ↑ Template:Cite web
- ↑
One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ 6.0 6.1
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 10.0 10.1
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534
