Outer product: Difference between revisions
en>Maschen remove merge banner, this has been declined by many |
en>Quondum removal of mixed <math> and HTML in single formula, {{nowrap}} for formulae & similar |
||
| Line 1: | Line 1: | ||
[[Image:Leidenfrost droplet.svg|320px|thumb|right|Leidenfrost droplet]] | |||
The '''Leidenfrost effect''' is a [[phenomenon]] in which a liquid, in near contact with a mass significantly hotter than the liquid's [[boiling point]], produces an insulating [[vapor]] layer keeping that liquid from [[boiling]] rapidly. This is most commonly seen when [[cooking]]; one sprinkles drops of water in a pan to gauge its temperature: if the pan's [[temperature]] is at or above the ''[[Leidenfrost point]]'', the water [[wikt:skitter|skitter]]s across the pan and takes longer to evaporate than in a pan below the temperature of the Leidenfrost point (but still above boiling temperature). The effect is also responsible for the ability of liquid nitrogen to skitter across floors. It has also been used in some potentially dangerous demonstrations, such as dipping a ''wet'' finger in molten [[lead]]<ref>http://www.csicop.org/si/9911/willey.html</ref> or blowing out a mouthful of [[liquid nitrogen]], both enacted without injury to the demonstrator.<ref>http://www.wiley.com/college/phy/halliday320005/pdf/leidenfrost_essay.pdf</ref> The latter is potentially lethal, particularly should one accidentally swallow the liquid nitrogen.<ref>http://www.wpi.edu/news/19989/nitro.html</ref> | |||
It is named after [[Johann Gottlob Leidenfrost]], who discussed it in ''A Tract About Some Qualities of Common Water'' in 1756. | |||
==Effect== | |||
[[File:Effet leidenfrost.ogg|right|thumb|right|A video clip demonstrating the Leidenfrost effect]] | |||
[[File:Spherical harmonic in water drop.ogv|thumb|Excitation of [[normal modes]] in a drop of water during the Leidenfrost effect]] | |||
The effect can be seen as drops of water are sprinkled onto a pan at various times as it heats up. Initially, as the temperature of the pan is below {{convert|100|C|F}}, the water just flattens out and slowly evaporates. As the temperature of the pan goes above {{convert|100|C|F}}, the water drops hiss when touching the pan and evaporate quickly. Later, as the temperature exceeds the Leidenfrost point, the Leidenfrost effect comes into play. On contact with the pan, the water droplets bunch up into small balls of water and skitter around, lasting much longer than when the temperature of the pan was lower. This effect works until a much higher temperature causes any further drops of water to evaporate too quickly to cause this effect. | |||
This is because at temperatures above the Leidenfrost point, the bottom part of the water droplet vaporizes immediately on contact with the hot plate. The resulting gas suspends the rest of the water droplet just above it, preventing any further direct contact between the liquid water and the hot plate. As steam has much poorer [[thermal conductivity]], further heat transfer between the pan and the droplet is slowed down dramatically. This also results in the drop being able to skid around the pan on the layer of gas just under it. | |||
[[File:Heat transfer leading to Leidenfrost effect for water at 1 atm.png|thumb|left|400 px|Behavior of water on a hot plate. Graph shows heat transfer (flux) vs temperature. Leidenfrost effect occurs after transition boiling.]] | |||
The temperature at which the Leidenfrost effect begins to occur is not easy to predict. Even if the volume of the drop of liquid stays the same, the Leidenfrost point may be quite different, with a complicated dependence on the properties of the surface, as well as any impurities in the liquid. Some research has been conducted into a theoretical model of the system, but it is quite complicated.<ref name="Bernardin & Mudawar" >Bernardin and Mudawar, "A Cavity Activation and Bubble Growth Model of the Leidenfrost Point," Transactions of the ASME, (Vol. 124, Oct. 2002)</ref> As a very rough estimate, the Leidenfrost point for a drop of water on a frying pan might occur at {{convert|193|C|F}}.{{Citation needed|date=August 2010}} | |||
The effect was also described by the eminent Victorian steam boiler designer, [[William Fairbairn|Sir William Fairbairn]], in reference to its effect on massively reducing heat transfer from a hot iron surface to water, such as within a boiler. In a pair of lectures on boiler design,<ref name="Fairbairn" >{{cite book |title=Two Lectures: The Construction of Boilers, and on Boiler Explosions, with the means of prevention |author=[[William Fairbairn|Sir William Fairbairn]] |year=1851 |url= http://books.google.co.uk/books?id=VD5MAAAAMAAJ&dq=fairbairn%20boiler&pg=PA1#v=onepage&q&f=false }}</ref> he cited the work of Pierre Hippolyte Boutigny (1798-1884) and Professor Bowman of [[King's College, London]] in studying this. A drop of water that was vaporized almost immediately at {{convert|334|F|C}} persisted for 152 seconds at {{convert|395|F|C}}. Lower temperatures in a boiler firebox might evaporate water ''more'' quickly as a result; compare [[Mpemba effect]]. An alternative approach was to increase the temperature beyond the Leidenfrost point. Fairbairn considered this too, and may have been contemplating the [[flash boiler|flash steam boiler]], but considered the technical aspects insurmountable for the time. | |||
The Leidenfrost point may also be taken to be the temperature for which the hovering droplet lasts longest.<ref name="ReferenceA">Incropera, DeWitt, Bergman & Lavine: Fundamentals of Heat and Mass Transfer, 6th edition.</ref> | |||
It has been demonstrated that it is possible to stabilize the Leidenfrost vapour layer of water by exploiting [[superhydrophobic]] surfaces. In this case, once the vapour layer is established, cooling never collapses the layer, and no nucleate boiling occurs; the layer instead slowly relaxes until the surface is cooled.<ref name="vakarelski2012">{{cite doi|10.1038/nature11418}}</ref> | |||
==Leidenfrost point== | |||
[[File:Water droplet Leidenfrost effect cropped.JPG|220px|thumb|right|A water droplet experiencing Leidenfrost effect on a hot stove hot plate]] | |||
The Leidenfrost point signifies the onset of stable film boiling. It represents the point on the boiling curve where the heat flux is at the minimum and the surface is completely covered by a vapor blanket. Heat transfer from the surface to the liquid occurs by conduction and radiation through the vapor. In 1756, Leidenfrost observed that water droplets supported by the vapor film slowly evaporate as they move about on the hot surface. As the surface temperature is increased, radiation through the vapor film becomes more significant and the heat flux increases with increasing excess temperature. | |||
The minimum heat flux for a large horizontal plate can be derived from Zuber's equation,<ref name="ReferenceA"/> | |||
<math>{{\frac{q}{A}}_{min}}=C{{h}_{fg}}{{\rho }_{v}}{{\left[ \frac{\sigma g\left( {{\rho }_{L}}-{{\rho }_{v}} \right)}{{{\left( {{\rho }_{L}}+{{\rho }_{v}} \right)}^{2}}} \right]}^{{}^{1}\!\!\diagup\!\!{}_{4}\;}}</math> | |||
where the properties are evaluated at saturation temperature. Zuber's constant, C is approximately 0.09 for most fluids at moderate pressures. | |||
==Heat transfer correlations== | |||
The heat transfer coefficient may be approximated using Bromley's equation,<ref name="ReferenceA"/> | |||
<math>h=C{{\left[ \frac{k_{v}^{3}{{\rho }_{v}}g\left( {{\rho }_{L}}-{{\rho }_{v}} \right)\left( {{h}_{fg}}+0.4{{c}_{pv}}\left( {{T}_{s}}-{{T}_{sat}} \right) \right)}{{{D}_{o}}{{\mu }_{v}}\left( {{T}_{s}}-{{T}_{sat}} \right)} \right]}^{{}^{1}\!\!\diagup\!\!{}_{4}\;}}</math> | |||
Where, <math>{{D}_{o}}</math> is the outside diameter of the tube. The correlation constant C is 0.62 for horizontal cylinders and vertical plates and 0.67 for spheres. Vapor properties are evaluated at film temperature. | |||
For stable film boiling on a horizontal surface, Berenson has modified Bromley's equation to yield,<ref name="ReferenceB">James R. Welty; Charles E. Wicks; Robert E. Wilson; Gregory L. Rorrer., "Fundamentals of Momentum, Heat and Mass transfer" 5th edition, John Wiley and Sons</ref> | |||
<math>h=0.425{{\left[ \frac{k_{vf}^{3}{{\rho }_{vf}}g\left( {{\rho }_{L}}-{{\rho }_{v}} \right)\left( {{h}_{fg}}+0.4{{c}_{pv}}\left( {{T}_{s}}-{{T}_{sat}} \right) \right)}{{{\mu }_{vf}}\left( {{T}_{s}}-{{T}_{sat}} \right)\sqrt{\sigma /g\left( {{\rho }_{L}}-{{\rho }_{v}} \right)}} \right]}^{{}^{1}\!\!\diagup\!\!{}_{4}\;}}</math> | |||
For vertical tubes, Hsu and Westwater have correlated the following equation,<ref name="ReferenceB"/> | |||
<math>h{{\left[ \frac{\mu _{v}^{2}}{g{{\rho }_{v}}\left( {{\rho }_{L}}-{{\rho }_{v}} \right)k_{v}^{3}} \right]}^{{}^{1}\!\!\diagup\!\!{}_{3}\;}}=0.0020{{\left[ \frac{4m}{\pi {{D}_{v}}{{\mu }_{v}}} \right]}^{0.6}}</math> | |||
Where, m is the mass flow rate in <math>l{{b}_{m}}/hr</math> at the upper end of the tube | |||
At excess temperatures above that at the minimum heat flux, the contribution of radiation becomes appreciable and becomes dominant at high excess temperatures. The total heat transfer coefficient can be is thus a combination of the two. Bromley has suggested the following equations for film boiling boiling from the outer surface of horizontal tubes. | |||
<math>{{h}^{{}^{4}\!\!\diagup\!\!{}_{3}\;}}={{h}_{conv}}^{{}^{4}\!\!\diagup\!\!{}_{3}\;}+{{h}_{rad}}{{h}^{{}^{1}\!\!\diagup\!\!{}_{3}\;}}</math> | |||
If <math>{{h}_{rad}}<{{h}_{conv}}</math>, | |||
<math>h={{h}_{conv}}+\frac{3}{4}{{h}_{rad}}</math> | |||
The effective radiation coefficient, <math>{{h}_{rad}}</math> can be expressed as, | |||
<math>{{h}_{rad}}=\frac{\varepsilon \sigma \left( T_{s}^{4}-T_{sat}^{4} \right)}{\left( {{T}_{s}}-{{T}_{sat}} \right)}</math> | |||
Where, <math>\varepsilon </math> is the emissivity of the solid and <math>\sigma </math> is the Stefan-Boltzmann constant. | |||
==In popular culture== | |||
In the 2009 season finale of ''[[MythBusters]]'', "[[MythBusters (2009 season)#Lead Plunge|Mini Myth Mayhem]]", the team demonstrated that a person can wet their hand and briefly dip it into molten [[lead]] without injury, using the Leidenfrost effect as the scientific basis.{{fact|date=November 2013}} | |||
==See also== | |||
* [[Critical heat flux]] | |||
* [[Mpemba effect]] | |||
* [[Nucleate boiling]] | |||
* [[region-beta paradox]] | |||
==References== | |||
{{Reflist}} | |||
==External links== | |||
{{Commonscat|Leidenfrost effect}} | |||
*[http://www.wiley.com/legacy/college/phy/halliday320005/pdf/leidenfrost_essay.pdf Essay about the effect and demonstrations by Jearl Walker] (PDF) | |||
*[http://www.uoregon.edu/~linke/climbingdroplets/index.html Site with high-speed video, pictures and explanation of film-boiling] by Heiner Linke at the University of Oregon, USA | |||
*[http://news.bbc.co.uk/1/hi/sci/tech/4955398.stm "Scientists make water run uphill"] by BBC News about using the Leidenfrost effect for cooling of [[computer chip]]s. | |||
* [http://www.abc.net.au/catalyst/stories/s1647557.htm "Uphill Water"] - ABC Catalyst story | |||
* [http://www.bath.ac.uk/news/2013/09/05/leidenfrost-maze/ "Leidenfrost Maze"] - University of Bath undergraduate students Carmen Cheng and Matthew Guy | |||
* [http://www.youtube.com/watch?v=zzKgnNGqxMw "When Water Flows Uphill"] - Science Friday with Univ of Bath professor Kei Takashina | |||
{{States of matter}} | |||
{{DEFAULTSORT:Leidenfrost Effect}} | |||
[[Category:Physical phenomena]] | |||
[[Category:Heat transfer]] | |||
Revision as of 17:37, 6 October 2013
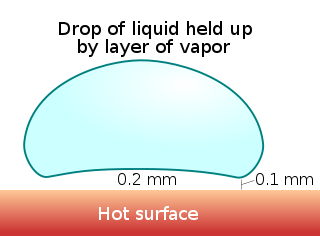
The Leidenfrost effect is a phenomenon in which a liquid, in near contact with a mass significantly hotter than the liquid's boiling point, produces an insulating vapor layer keeping that liquid from boiling rapidly. This is most commonly seen when cooking; one sprinkles drops of water in a pan to gauge its temperature: if the pan's temperature is at or above the Leidenfrost point, the water skitters across the pan and takes longer to evaporate than in a pan below the temperature of the Leidenfrost point (but still above boiling temperature). The effect is also responsible for the ability of liquid nitrogen to skitter across floors. It has also been used in some potentially dangerous demonstrations, such as dipping a wet finger in molten lead[1] or blowing out a mouthful of liquid nitrogen, both enacted without injury to the demonstrator.[2] The latter is potentially lethal, particularly should one accidentally swallow the liquid nitrogen.[3]
It is named after Johann Gottlob Leidenfrost, who discussed it in A Tract About Some Qualities of Common Water in 1756.
Effect
File:Spherical harmonic in water drop.ogv
The effect can be seen as drops of water are sprinkled onto a pan at various times as it heats up. Initially, as the temperature of the pan is below Template:Convert, the water just flattens out and slowly evaporates. As the temperature of the pan goes above Template:Convert, the water drops hiss when touching the pan and evaporate quickly. Later, as the temperature exceeds the Leidenfrost point, the Leidenfrost effect comes into play. On contact with the pan, the water droplets bunch up into small balls of water and skitter around, lasting much longer than when the temperature of the pan was lower. This effect works until a much higher temperature causes any further drops of water to evaporate too quickly to cause this effect.
This is because at temperatures above the Leidenfrost point, the bottom part of the water droplet vaporizes immediately on contact with the hot plate. The resulting gas suspends the rest of the water droplet just above it, preventing any further direct contact between the liquid water and the hot plate. As steam has much poorer thermal conductivity, further heat transfer between the pan and the droplet is slowed down dramatically. This also results in the drop being able to skid around the pan on the layer of gas just under it.

The temperature at which the Leidenfrost effect begins to occur is not easy to predict. Even if the volume of the drop of liquid stays the same, the Leidenfrost point may be quite different, with a complicated dependence on the properties of the surface, as well as any impurities in the liquid. Some research has been conducted into a theoretical model of the system, but it is quite complicated.[4] As a very rough estimate, the Leidenfrost point for a drop of water on a frying pan might occur at Template:Convert.Potter or Ceramic Artist Truman Bedell from Rexton, has interests which include ceramics, best property developers in singapore developers in singapore and scrabble. Was especially enthused after visiting Alejandro de Humboldt National Park.
The effect was also described by the eminent Victorian steam boiler designer, Sir William Fairbairn, in reference to its effect on massively reducing heat transfer from a hot iron surface to water, such as within a boiler. In a pair of lectures on boiler design,[5] he cited the work of Pierre Hippolyte Boutigny (1798-1884) and Professor Bowman of King's College, London in studying this. A drop of water that was vaporized almost immediately at Template:Convert persisted for 152 seconds at Template:Convert. Lower temperatures in a boiler firebox might evaporate water more quickly as a result; compare Mpemba effect. An alternative approach was to increase the temperature beyond the Leidenfrost point. Fairbairn considered this too, and may have been contemplating the flash steam boiler, but considered the technical aspects insurmountable for the time.
The Leidenfrost point may also be taken to be the temperature for which the hovering droplet lasts longest.[6]
It has been demonstrated that it is possible to stabilize the Leidenfrost vapour layer of water by exploiting superhydrophobic surfaces. In this case, once the vapour layer is established, cooling never collapses the layer, and no nucleate boiling occurs; the layer instead slowly relaxes until the surface is cooled.[7]
Leidenfrost point

The Leidenfrost point signifies the onset of stable film boiling. It represents the point on the boiling curve where the heat flux is at the minimum and the surface is completely covered by a vapor blanket. Heat transfer from the surface to the liquid occurs by conduction and radiation through the vapor. In 1756, Leidenfrost observed that water droplets supported by the vapor film slowly evaporate as they move about on the hot surface. As the surface temperature is increased, radiation through the vapor film becomes more significant and the heat flux increases with increasing excess temperature.
The minimum heat flux for a large horizontal plate can be derived from Zuber's equation,[6]
where the properties are evaluated at saturation temperature. Zuber's constant, C is approximately 0.09 for most fluids at moderate pressures.
Heat transfer correlations
The heat transfer coefficient may be approximated using Bromley's equation,[6]
Where, is the outside diameter of the tube. The correlation constant C is 0.62 for horizontal cylinders and vertical plates and 0.67 for spheres. Vapor properties are evaluated at film temperature.
For stable film boiling on a horizontal surface, Berenson has modified Bromley's equation to yield,[8]
For vertical tubes, Hsu and Westwater have correlated the following equation,[8]
Where, m is the mass flow rate in at the upper end of the tube
At excess temperatures above that at the minimum heat flux, the contribution of radiation becomes appreciable and becomes dominant at high excess temperatures. The total heat transfer coefficient can be is thus a combination of the two. Bromley has suggested the following equations for film boiling boiling from the outer surface of horizontal tubes.
The effective radiation coefficient, can be expressed as,
Where, is the emissivity of the solid and is the Stefan-Boltzmann constant.
In popular culture
In the 2009 season finale of MythBusters, "Mini Myth Mayhem", the team demonstrated that a person can wet their hand and briefly dip it into molten lead without injury, using the Leidenfrost effect as the scientific basis.Template:Fact
See also
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
External links
- Essay about the effect and demonstrations by Jearl Walker (PDF)
- Site with high-speed video, pictures and explanation of film-boiling by Heiner Linke at the University of Oregon, USA
- "Scientists make water run uphill" by BBC News about using the Leidenfrost effect for cooling of computer chips.
- "Uphill Water" - ABC Catalyst story
- "Leidenfrost Maze" - University of Bath undergraduate students Carmen Cheng and Matthew Guy
- "When Water Flows Uphill" - Science Friday with Univ of Bath professor Kei Takashina
- ↑ http://www.csicop.org/si/9911/willey.html
- ↑ http://www.wiley.com/college/phy/halliday320005/pdf/leidenfrost_essay.pdf
- ↑ http://www.wpi.edu/news/19989/nitro.html
- ↑ Bernardin and Mudawar, "A Cavity Activation and Bubble Growth Model of the Leidenfrost Point," Transactions of the ASME, (Vol. 124, Oct. 2002)
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 6.0 6.1 6.2 Incropera, DeWitt, Bergman & Lavine: Fundamentals of Heat and Mass Transfer, 6th edition.
- ↑ Template:Cite doi
- ↑ 8.0 8.1 James R. Welty; Charles E. Wicks; Robert E. Wilson; Gregory L. Rorrer., "Fundamentals of Momentum, Heat and Mass transfer" 5th edition, John Wiley and Sons
![{\displaystyle {{\frac {q}{A}}_{min}}=C{{h}_{fg}}{{\rho }_{v}}{{\left[{\frac {\sigma g\left({{\rho }_{L}}-{{\rho }_{v}}\right)}{{\left({{\rho }_{L}}+{{\rho }_{v}}\right)}^{2}}}\right]}^{{}^{1}\!\!\diagup \!\!{}_{4}\;}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a94fba1cc42308142bf2448820f0cd14a1fdb86d)
![{\displaystyle h=C{{\left[{\frac {k_{v}^{3}{{\rho }_{v}}g\left({{\rho }_{L}}-{{\rho }_{v}}\right)\left({{h}_{fg}}+0.4{{c}_{pv}}\left({{T}_{s}}-{{T}_{sat}}\right)\right)}{{{D}_{o}}{{\mu }_{v}}\left({{T}_{s}}-{{T}_{sat}}\right)}}\right]}^{{}^{1}\!\!\diagup \!\!{}_{4}\;}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3f3d23ccc06ec9190f361d504cd6b982deeea09c)

![h=0.425{{\left[{\frac {k_{{vf}}^{{3}}{{\rho }_{{vf}}}g\left({{\rho }_{{L}}}-{{\rho }_{{v}}}\right)\left({{h}_{{fg}}}+0.4{{c}_{{pv}}}\left({{T}_{{s}}}-{{T}_{{sat}}}\right)\right)}{{{\mu }_{{vf}}}\left({{T}_{{s}}}-{{T}_{{sat}}}\right){\sqrt {\sigma /g\left({{\rho }_{{L}}}-{{\rho }_{{v}}}\right)}}}}\right]}^{{{}^{{1}}\!\!\diagup \!\!{}_{{4}}\;}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f0c9bf294fc90d5b0b54986a8bd1b4684966917b)
![{\displaystyle h{{\left[{\frac {\mu _{v}^{2}}{g{{\rho }_{v}}\left({{\rho }_{L}}-{{\rho }_{v}}\right)k_{v}^{3}}}\right]}^{{}^{1}\!\!\diagup \!\!{}_{3}\;}}=0.0020{{\left[{\frac {4m}{\pi {{D}_{v}}{{\mu }_{v}}}}\right]}^{0.6}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/315bcfe771ba9265972a7431972f7de1ab2fd75b)







