Pohlig–Hellman algorithm: Difference between revisions
Jump to navigation
Jump to search
en>YFdyh-bot m r2.7.3) (Robot: Adding uk:Алгоритм Поліґа-Геллмана |
|||
| Line 1: | Line 1: | ||
<!-- To obtain a blank version of this page, type <nowiki>{{chembox supplement}}</nowiki> and save the page --> | |||
This page provides supplementary chemical data on [[acetic acid]]. | |||
== Material Safety Data Sheet == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet ([[Material safety data sheet|MSDS]]) for this chemical from a reliable source and follow its directions. | |||
*[http://web.archive.org/web/20090221184944/http://www.jtbaker.com/msds/englishhtml/a5508.htmhttp://www2.siri.org/msds/index.php SIRI] | |||
*[http://ptcl.chem.ox.ac.uk/MSDS/AC/acetic_acid.html PTCL Safety web site] | |||
*[http://www.sciencestuff.com/msds/C1120.html Science Stuff] | |||
== Structure and properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Structure and properties | |||
|- | |||
| [[Index of refraction]], ''n''<sub>D</sub> | |||
| 1.3716 | |||
|- | |||
| [[Dielectric constant]], ε<sub>r</sub> | |||
| 6.15 ε<sub>0</sub> at 20 °C <!-- Please omit if not applicable --> | |||
|- | |||
| [[Bond strength (chemistry)|Bond strength]] | |||
| ? <!-- Specify which bond. Please omit if not applicable --> | |||
|- | |||
| [[Bond length]] | |||
| ? <!-- Specify which bond. Please omit if not applicable --> | |||
|- | |||
| [[Bond angle]] | |||
| ? <!-- Specify which angle, e.g. Cl-P-O. Please omit if not applicable --> | |||
|- | |||
| [[Magnetic susceptibility]] | |||
| ? <!-- Please omit if not applicable --> | |||
|- | |||
| [[Surface tension]] | |||
| 26.6 dyn/cm at 30°C | |||
|- | |||
| [[Viscosity]]<ref name="lange_1669">''Lange's Handbook of Chemistry'', 10th ed. pp 1669-1674</ref> | |||
| | |||
{| | |||
| 1.222 mPa·s || at 20°C | |||
|- | |||
| 1.0396 mPa·s || at 30°C | |||
|- | |||
| 0.7956 mPa·s || at 50°C | |||
|- | |||
| 0.4244 mPa·s || at 110°C | |||
|} | |||
|- | |||
|} | |||
== Thermodynamic properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Phase behavior | |||
|- | |||
| [[Triple point]] | |||
| 289.8 K (16.7 °C), ? Pa | |||
|- | |||
| [[Critical point (thermodynamics)|Critical point]] | |||
| 593 K (320 °C), 57.8 bar | |||
|- | |||
| [[Eutectic point]] with water | |||
| –26.7 °C | |||
|- | |||
| [[Standard enthalpy change of fusion|Std enthalpy change<br/>of fusion]]Δ<sub>fus</sub>''H''<sup><s>o</s></sup> | |||
| +11.7 kJ/mol | |||
|- | |||
| [[Standard entropy change of fusion|Std entropy change<br/>of fusion]]Δ<sub>fus</sub>''S''<sup><s>o</s></sup> | |||
| 40.5 J/(mol·K) | |||
|- | |||
| [[Standard enthalpy change of vaporization|Std enthalpy change<br/>of vaporization]]Δ<sub>vap</sub>''H''<sup><s>o</s></sup> | |||
| +23.7 kJ/mol | |||
|- | |||
| [[Standard entropy change of vaporization|Std entropy change<br/>of vaporization]]Δ<sub>vap</sub>''S''<sup><s>o</s></sup> | |||
| ? J/(mol·K) | |||
|- | |||
! {{chembox header}} | Solid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]] Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>solid</sub> | |||
| ? kJ/mol | |||
|- | |||
| [[Standard molar entropy]]<br/>''S''<sup><s>o</s></sup><sub>solid</sub> | |||
| ? J/(mol K) | |||
|- | |||
| [[Heat capacity]] ''c<sub>p</sub>'' | |||
| ? J/(mol K) | |||
|- | |||
! {{chembox header}} | Liquid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]] Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>liquid</sub> | |||
| −483.5 kJ/mol | |||
|- | |||
| [[Standard molar entropy]]<br/>''S''<sup><s>o</s></sup><sub>liquid</sub> | |||
| 158.0 J/(mol K) | |||
|- | |||
| [[Enthalpy of combustion]], Δ<sub>c</sub>''H''<sup><s>o</s></sup><sub> | |||
| –876.1 kJ/mol | |||
|- | |||
| [[Heat capacity]] ''c<sub>p</sub>'' | |||
| 123.1 J/(mol K) | |||
|- | |||
! {{chembox header}} | Gas properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]] Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>gas</sub> | |||
| –438.1 kJ/mol | |||
|- | |||
| [[Standard molar entropy]]<br/>''S''<sup><s>o</s></sup><sub>gas</sub> | |||
| 195 J/(mol K) | |||
|- | |||
| [[Heat capacity]] ''c<sub>p</sub>'' | |||
| 63.4 J/(mol K) | |||
|- | |||
| [[van der Waals equation|van der Waals' constants]]<ref name="lange1522">''Lange's Handbook of Chemistry'' 10th ed, pp 1522-1524</ref> | |||
| a = 1782.3 L<sup>2</sup> kPa/mol<sup>2</sup><br> b = 0.1068 liter per mole | |||
|- | |||
|} | |||
==Vapor pressure of liquid== | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| {{chembox header}} | '''P in mm Hg''' || 1 || 10 || 40 || 100 || 400 || 760 || 1520 || 3800 || 7600 || 15200 || 30400|| 45600 | |||
|- | |||
| {{chembox header}} | '''T in °C''' || –17.2 || 17.5 || 43.0 || 63.0 || 99.0 || 118.1 || 143.5 || 180.3 || 214.0 || 252.0 || 297.0 || — | |||
|} | |||
Table data obtained from ''CRC Handbook of Chemistry and Physics'' 44th ed. | |||
{| | |||
| | |||
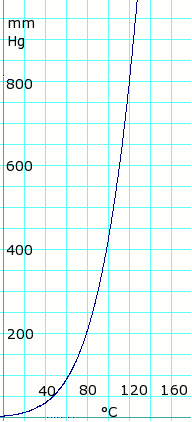
[[Image:aceticAcidVaporPressure.png|thumb|192px|left|Acetic acid vapor pressure vs. temperature. Uses formula: <math>\scriptstyle P_{mmHg}=10^{7.80307 - \frac {1651.2} {225+T}}</math> for T = 0 to 36°C | |||
<math>\scriptstyle P_{mmHg}=10^{7.18807 - \frac {1416.7} {211+T}}</math> for T = 36 to 170°C<br> | |||
Formula from ''Lange's Handbook of Chemistry'', 10th ed.]] | |||
| | |||
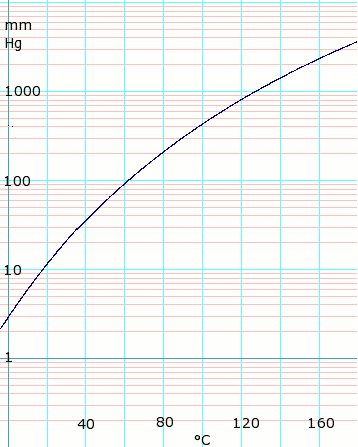
[[Image:logAceticAcidVaporPressure.png|thumb|358px|left|log<sub>10</sub> of acetic acid vapor pressure vs. temperature. Uses formula: <math>\scriptstyle \log_{10}P_{mmHg}=7.80307 - \frac {1651.2} {225+T}</math> for T = 0 to 36°C | |||
| |||
<math>\scriptstyle \log_{10}P_{mmHg}=7.18807 - \frac {1416.7} {211+T}</math> for T = 36 to 170°C<br> | |||
Formula from ''Lange's Handbook of Chemistry'', 10th ed. | |||
]] | |||
|} | |||
==Distillation data== | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| bgcolor="#D0D0D0" align="center" colspan="3"| '''Vapor-liquid Equilibrium for Acetic acid/Water'''<ref>{{cite web|url=http://www.cheric.org/research/kdb/hcvle/hcvle.php|title=Binary Vapor-Liquid Equilibrium Data|publisher=Chemical Engineering Research Information Center|format=Queriable database|accessdate=5 May 2007}}</ref><br>''P'' = 760 mm Hg | |||
|- {{chembox header}} | |||
! rowspan="2" | BP<br>Temp.<br>°C | |||
! colspan="2" | % by mole water | |||
|- {{chembox header}} | |||
! liquid !! vapor | |||
|- | |||
| 116.5 || 2.2 || 5.8 | |||
|- | |||
| 114.6 || 5.4 || 12.3 | |||
|- | |||
| 113.4 || 8.6 || 16.8 | |||
|- | |||
| 113.5 || 9.9 || 18.3 | |||
|- | |||
| 113.1 || 10.1 || 18.8 | |||
|- | |||
| 110.6 || 18.9 || 29.8 | |||
|- | |||
| 107.8 || 30.3 || 43.3 | |||
|- | |||
| 106.1 || 41.3 || 54.5 | |||
|- | |||
| 104.4 || 52.2 || 64.9 | |||
|- | |||
| 103.1 || 62.4 || 73.5 | |||
|- | |||
| 102.3 || 69.6 || 79.2 | |||
|- | |||
| 101.6 || 77.8 || 85.1 | |||
|- | |||
| 100.8 || 87.6 || 91.4 | |||
|- | |||
| 100.5 || 92.3 || 94.4 | |||
|- | |||
| 100.4 || 94.5 || 96.0 | |||
|- | |||
| 100.1 || 98.5 || 98.9 | |||
|- | |||
|} | |||
== Spectral data == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | [[UV/VIS spectroscopy|UV-Vis]] | |||
|- | |||
| [[Lambda-max|λ<sub>max</sub>]] | |||
| 207 [[Nanometre|nm]] (gas phase) | |||
|- | |||
| [[molar absorptivity|Extinction coefficient]], ε | |||
| ? | |||
|- | |||
! {{chembox header}} | [[Infrared|IR]] | |||
|- | |||
| Major absorption bands<ref name="aist">{{cite web|url=http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng|title=Spectral Database for Organic Compounds|publisher=Advanced Industrial Science and Technology|format=Queriable database|accessdate=9 June 2007}}</ref> | |||
| | |||
{| | |||
|- | |||
| colspan="2" align="center" | (liquid film) | |||
|- | |||
! Wave number !! transmittance | |||
|- | |||
| 2937 cm<sup>−1</sup> || 26% | |||
|- | |||
| 2684 cm<sup>−1</sup> || 41% | |||
|- | |||
| 2631 cm<sup>−1</sup> || 39% | |||
|- | |||
| 2569 cm<sup>−1</sup> || 49% | |||
|- | |||
| 1758 cm<sup>−1</sup> || 19% | |||
|- | |||
| 1714 cm<sup>−1</sup> || 4% | |||
|- | |||
| 1617 cm<sup>−1</sup> || 66% | |||
|- | |||
| 1414 cm<sup>−1</sup> || 20% | |||
|- | |||
| 1360 cm<sup>−1</sup> || 39% | |||
|- | |||
| 1294 cm<sup>−1</sup> || 12% | |||
|- | |||
| 1053 cm<sup>−1</sup> || 67% | |||
|- | |||
| 1016 cm<sup>−1</sup> || 41% | |||
|- | |||
| 937 cm<sup>−1</sup> || 35% | |||
|- | |||
| 892 cm<sup>−1</sup> || 41% | |||
|- | |||
| 629 cm<sup>−1</sup> || 31% | |||
|- | |||
| 607 cm<sup>−1</sup> || 49% | |||
|- | |||
| 481 cm<sup>−1</sup> || 36% | |||
|- | |||
| 473 cm<sup>−1</sup> || 52% | |||
|- | |||
|} | |||
|- | |||
! {{chembox header}} | [[NMR Spectroscopy|NMR]] | |||
|- | |||
| [[Proton NMR]] <!-- Link to image of spectrum --> | |||
| δ CDCl<sub>3</sub> 2.10 (3H), 11.42 (1H) | |||
|- | |||
| [[Carbon-13 NMR]] <!-- Link to image of spectrum --> | |||
| δ CDCl<sub>3</sub> 20.8, 178.1 | |||
|- | |||
| Other NMR data <!-- Insert special data e.g. <sup>19</sup>F chem. shifts, omit if not used --> | |||
| | |||
|- | |||
! {{chembox header}} | [[Mass Spectrometry|MS]] | |||
|- | |||
| Masses of <br>main fragments | |||
| 60 (75%), 45 (90%),<br/>43 (100%), 42 (13%), 15 (17%) | |||
|- | |||
|} | |||
== References == | |||
{{reflist}} | |||
*{{nist}} | |||
*{{Cite journal | doi = 10.1016/S1010-6030(03)00067-4 | last1 = Orlando | first1 = John J. | last2 = Tyndall | first2 = Geoffrey S. | year = 2003 | title = Gas phase UV absorption spectra for peracetic acid, and for acetic acid monomers and dimers | url = http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TGY-483STKX-8&_user=10&_handle=V-WA-A-W-WW-MsSAYVW-UUW-U-AABVVDCEED-AABWUCZDED-VDWUCAVDC-WW-U&_fmt=summary&_coverDate=05%2F05%2F2003&_rdoc=5&_orig=browse&_srch=%23toc%235267%232003%23998429997%23418946!&_cdi=5267&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=89de30203134fccccf77c80dce6b5ccf | journal = J. Photochem. Photobiol. A: Chem. | volume = 157 | issue = 2–3| pages = 161–66 }} | |||
Except where noted otherwise, data relate to [[standard ambient temperature and pressure]]. | |||
[[wikipedia:Chemical infobox|Disclaimer]] applies. | |||
{{DEFAULTSORT:Acetic Acid (Data Page)}} | |||
[[Category:Chemical data pages]] | |||
Revision as of 18:20, 2 December 2013
This page provides supplementary chemical data on acetic acid.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index of refraction, nD | 1.3716 | ||||||||
| Dielectric constant, εr | 6.15 ε0 at 20 °C | ||||||||
| Bond strength | ? | ||||||||
| Bond length | ? | ||||||||
| Bond angle | ? | ||||||||
| Magnetic susceptibility | ? | ||||||||
| Surface tension | 26.6 dyn/cm at 30°C | ||||||||
| Viscosity[1] |
|
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 289.8 K (16.7 °C), ? Pa |
| Critical point | 593 K (320 °C), 57.8 bar |
| Eutectic point with water | –26.7 °C |
| Std enthalpy change of fusionΔfusH |
+11.7 kJ/mol |
| Std entropy change of fusionΔfusS |
40.5 J/(mol·K) |
| Std enthalpy change of vaporizationΔvapH |
+23.7 kJ/mol |
| Std entropy change of vaporizationΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation ΔfH |
? kJ/mol |
| Standard molar entropy S |
? J/(mol K) |
| Heat capacity cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation ΔfH |
−483.5 kJ/mol |
| Standard molar entropy S |
158.0 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–876.1 kJ/mol |
| Heat capacity cp | 123.1 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation ΔfH |
–438.1 kJ/mol |
| Standard molar entropy S |
195 J/(mol K) |
| Heat capacity cp | 63.4 J/(mol K) |
| van der Waals' constants[2] | a = 1782.3 L2 kPa/mol2 b = 0.1068 liter per mole |
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 |
| T in °C | –17.2 | 17.5 | 43.0 | 63.0 | 99.0 | 118.1 | 143.5 | 180.3 | 214.0 | 252.0 | 297.0 | — |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
Distillation data
| Vapor-liquid Equilibrium for Acetic acid/Water[3] P = 760 mm Hg | ||
| BP Temp. °C |
% by mole water | |
|---|---|---|
| liquid | vapor | |
| 116.5 | 2.2 | 5.8 |
| 114.6 | 5.4 | 12.3 |
| 113.4 | 8.6 | 16.8 |
| 113.5 | 9.9 | 18.3 |
| 113.1 | 10.1 | 18.8 |
| 110.6 | 18.9 | 29.8 |
| 107.8 | 30.3 | 43.3 |
| 106.1 | 41.3 | 54.5 |
| 104.4 | 52.2 | 64.9 |
| 103.1 | 62.4 | 73.5 |
| 102.3 | 69.6 | 79.2 |
| 101.6 | 77.8 | 85.1 |
| 100.8 | 87.6 | 91.4 |
| 100.5 | 92.3 | 94.4 |
| 100.4 | 94.5 | 96.0 |
| 100.1 | 98.5 | 98.9 |
Spectral data
| UV-Vis | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax | 207 nm (gas phase) | ||||||||||||||||||||||||||||||||||||||||
| Extinction coefficient, ε | ? | ||||||||||||||||||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||||||||||||||||||
| Major absorption bands[4] |
| ||||||||||||||||||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||||||||||||||||||
| Proton NMR | δ CDCl3 2.10 (3H), 11.42 (1H) | ||||||||||||||||||||||||||||||||||||||||
| Carbon-13 NMR | δ CDCl3 20.8, 178.1 | ||||||||||||||||||||||||||||||||||||||||
| Other NMR data | |||||||||||||||||||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||||||||||||||||||
| Masses of main fragments |
60 (75%), 45 (90%), 43 (100%), 42 (13%), 15 (17%) | ||||||||||||||||||||||||||||||||||||||||
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
- Template:Nist
- One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
- ↑ Lange's Handbook of Chemistry, 10th ed. pp 1669-1674
- ↑ Lange's Handbook of Chemistry 10th ed, pp 1522-1524
- ↑ Template:Cite web
- ↑ Template:Cite web